Polyarylether sulphone resin with Si-O-Si structure in main chain and preparation method of resin
A technology of polyarylethersulfone and si-o-si, which is applied in the field of hydrophobic polyarylethersulfone resin and its preparation, to achieve the effects of lowering the glass transition temperature, improving solubility and hydrophobicity, and excellent heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 1,1,3,3-tetramethyldihydrodisiloxane (6.7g, 0.05mol) and eugenol (16.4g, 0.1mol) in a nitrogen-protected container, and add toluene (92mL) to the system As a solvent, heat up to 65°C, add 3~5 drops of chloroplatinic acid solution, react at constant temperature for 6~12 hours, then cool down to room temperature, add the reaction mixture into a vacuum distillation device, and distill under reduced pressure at 120°C to remove unreacted Eugenol and solvent toluene were cooled to obtain 18.7 g of a colorless viscous transparent liquid 2Si-PH with a yield of 80.9%.
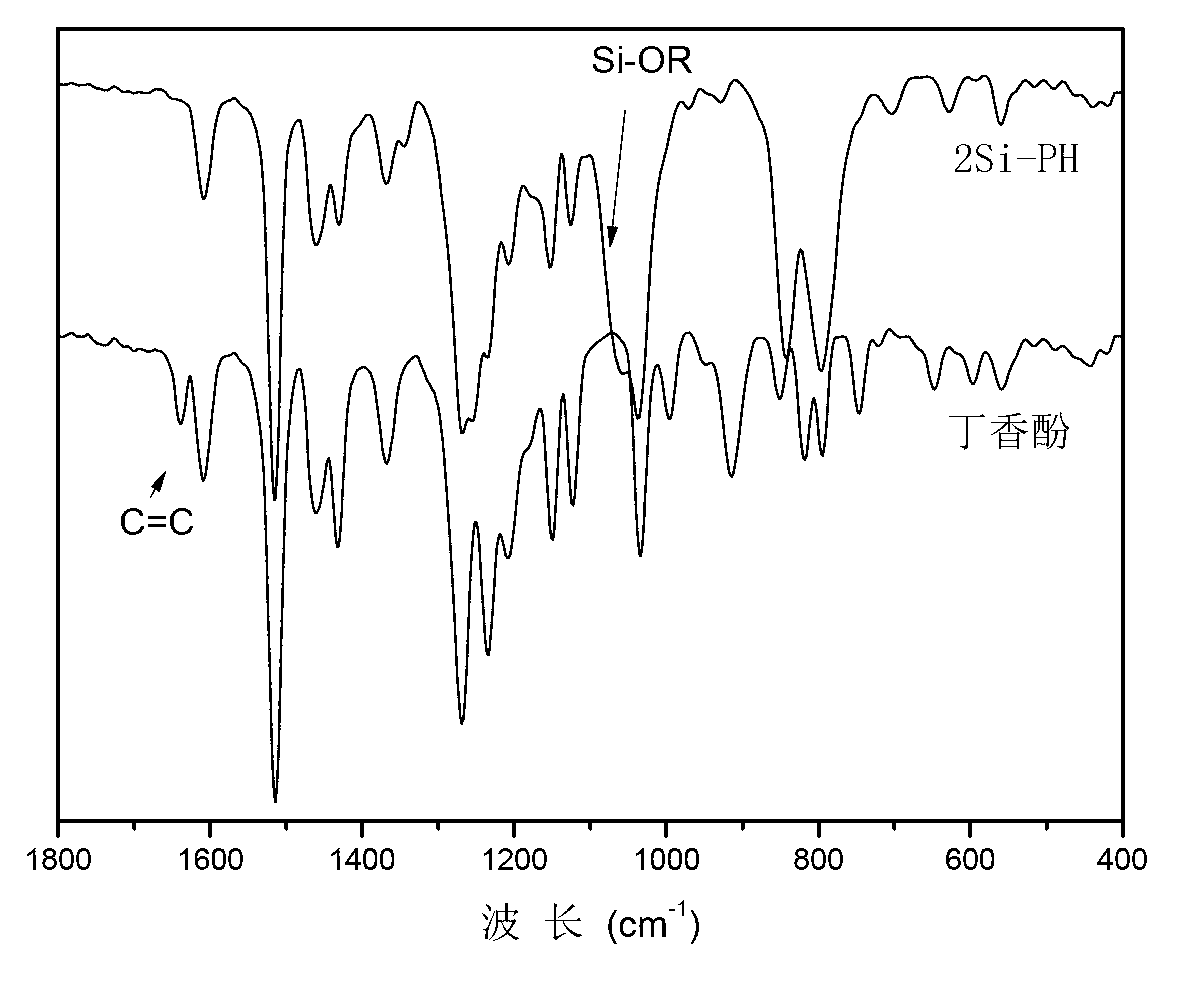
[0031] Use Nicolet Impact410 Fourier Transform Infrared Spectrometer to measure the infrared spectrum of 2Si-PH and eugenol, the scanning range is 4000~400cm -1 , resolution 4cm -1 , KBr tablet, see the results figure 1 . Depend on figure 1 It can be seen that the C=C bond absorption peak in eugenol has disappeared in 2Si-PH, and the characteristic peak of Si-O bond appears in the reacted 2Si-PH, indicati...
Embodiment 2
[0035] Add 2Si-PH monomer, biphenol, dichlorodiphenyl sulfone and sodium carbonate into a nitrogen-protected container, use sulfolane as a solvent, and use toluene as a water-carrying agent, heat up to 150°C, and react with water to form a salt for 4 hours . The temperature was raised to 180°C for 2 hours, the temperature was raised to 200°C for 2 hours, and then the temperature was raised to 220°C for 2 hours. Discharge in deionized water, pulverize, and wash with water and ethanol three times respectively to prepare polyarylethersulfone 1 and polyarylethersulfone 2-5 with main chain containing Si-O-Si structure. The feed ratio and production rate are shown in Table 1.
[0036] Table 1 The feed ratio and yield of polyarylethersulfone 1~5
[0037]
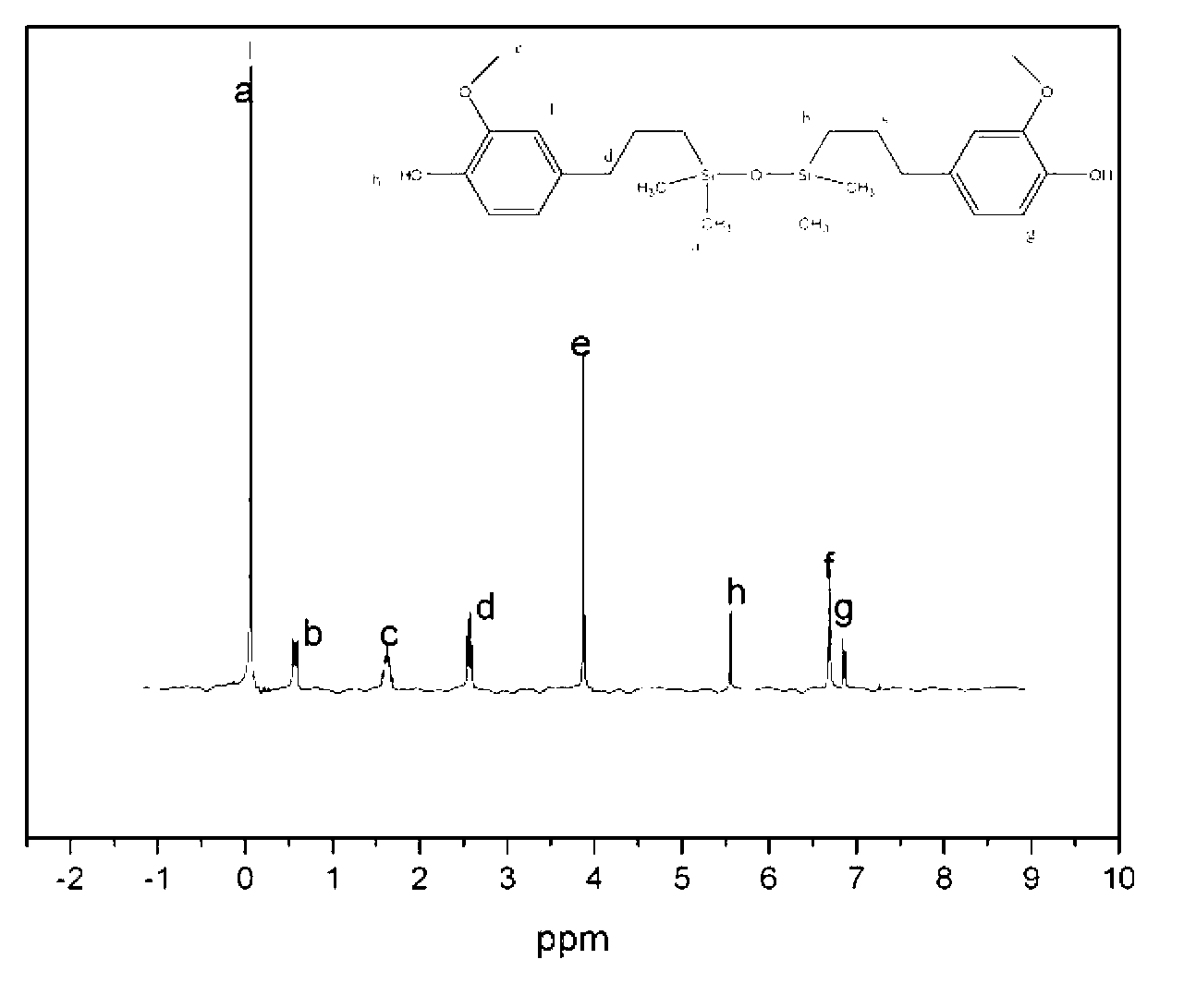
[0038] Bruker Advance 510 nuclear magnetic resonance instrument (500MHz) was used to measure the hydrogen nuclear magnetic resonance spectrum of polyarylethersulfone 1 and polyarylethersulfone 5 containing Si-O-Si structure in...
Embodiment 3
[0052] Polymerization was carried out by changing the feed ratio, and the synthesis process was the same as that in Example 2 to prepare polyarylethersulfone 6 and polyarylethersulfone 7-10 with a main chain containing Si-O-Si structure. The feed ratio and production rate are shown in Table 5.
[0053] Table 5 The feed ratio and yield of polyarylethersulfone 6~10
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com