Artificial simulation hydrogenase of aromatic ether dendritic polymer and application of artificial simulation hydrogenase
An artificial analog, dendritic technology, applied in the field of energy science and catalysis science, can solve the problem of hydrogen production activity gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
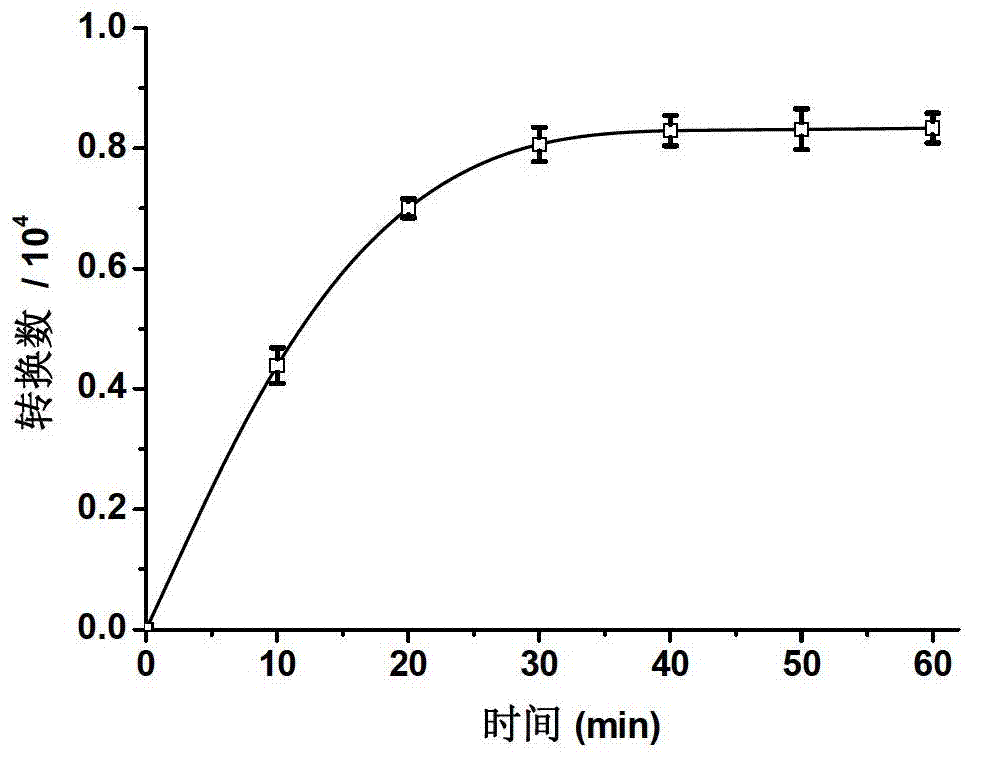
Embodiment 1
[0083] Synthesis of a first-generation bibranched aryl ether dendrimer artificially mimicking hydrogenase (Hy-G1):
[0084]
[0085] Add 1.0 mmol of [(μ-S 2 )Fe(CO) 6 ] and 10.0 mL of dry tetrahydrofuran, liquid nitrogen freezing-vacuumizing-nitrogen, repeat three times. Slowly drop 2.0mL containing 2.0mmol LiBHEt under nitrogen protection and -78℃ cooling bath 3 THF solution, flow rate 1ml / 5min. After the dropwise addition, keep stirring at -78°C for 10 minutes. Dissolve 2.0 mmol of first-generation benzyl bromide core aryl ether dendrimers (G1-Br) in 2.0 mL of dry tetrahydrofuran solution, and add to the above reaction solution after purging with nitrogen to remove oxygen. After the addition was completed, it was raised to room temperature, and the stirring reaction was continued for 5h. After the reaction, the solvent was removed under reduced pressure, and the crude product was separated by column chromatography (eluent: 5 / 2 dichloromethane / petroleum ether) to obta...
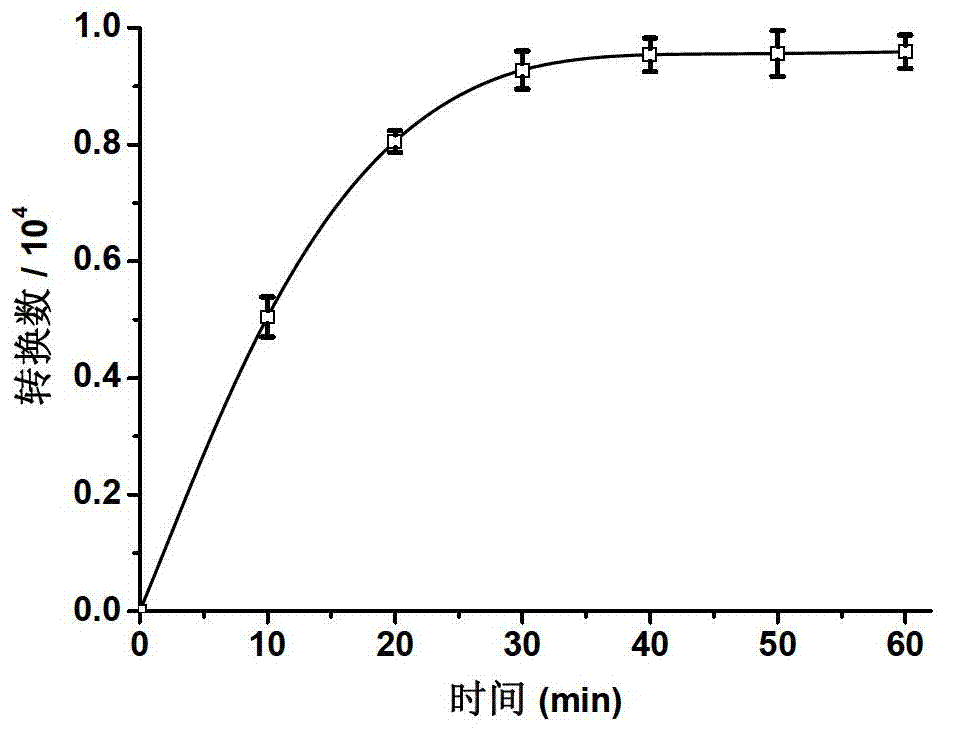
Embodiment 2
[0087] Synthesis of second-generation bibranched aryl ether dendrimers artificially mimicking hydrogenase (Hy-G2):
[0088]
[0089] Add 1.0 mmol of [(μ-S 2 )Fe(CO) 6 ] and 10.0 mL of dry tetrahydrofuran, liquid nitrogen freezing-vacuumizing-nitrogen, repeat three times. Slowly add 10.0mL of LiBHEt containing 2.0mmol LiBHEt 3 THF solution, flow rate 1ml / 5min. After the dropwise addition, keep stirring at -78°C for 10 minutes. Dissolve 2.0 mmol of aryl ether dendrimers (G2-Br) with a digeneration benzyl bromide core in 2.0 mL of dry tetrahydrofuran solution, and add to the above reaction solution after purging with nitrogen to remove oxygen. After the addition was completed, it was raised to room temperature, and the stirring reaction was continued for 5h. After the reaction, the solvent was removed under reduced pressure, and the crude product was separated by column chromatography (eluent: 5 / 2 dichloromethane / petroleum ether) to obtain a red glassy solid with a yield ...
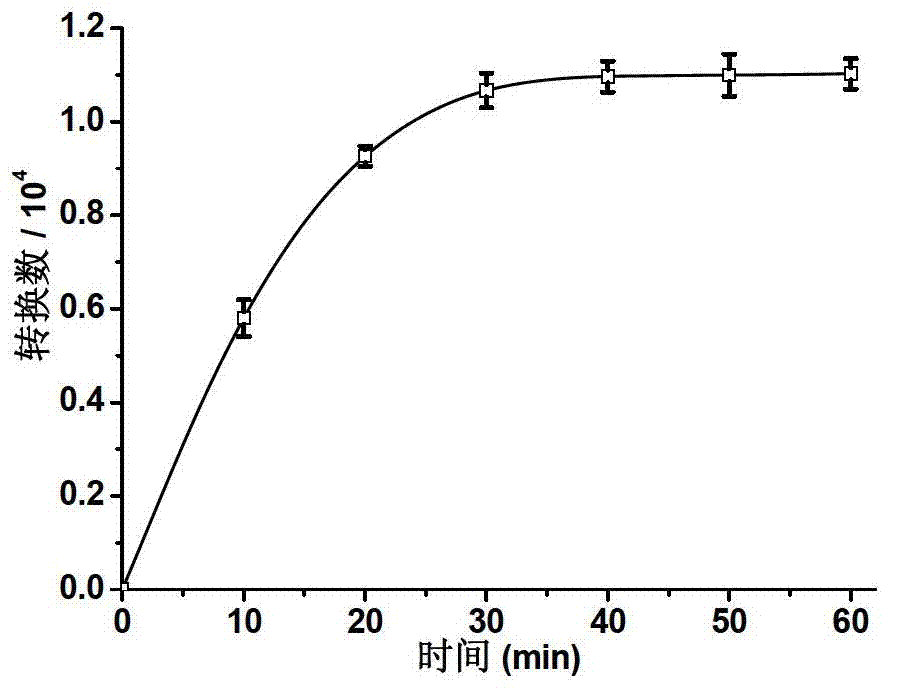
Embodiment 3
[0091] Synthesis of three-generation bis-branched aryl ether dendrimers artificially mimicking hydrogenase (Hy-G3):
[0092]
[0093] Add 1.0 mmol of [(μ-S 2 )Fe(CO) 6 ] and 10.0 mL of dry tetrahydrofuran, liquid nitrogen freezing-vacuumizing-nitrogen, repeat three times. Slowly add 10.0mL of LiBHEt containing 2.0mmol LiBHEt 3 THF solution, flow rate 1ml / 5min. After the dropwise addition, keep stirring at -78°C for 10 minutes. Dissolve 2.0 mmol of three-generation benzyl bromide-core aryl ether dendrimers (G3-Br) in 2.0 mL of dry tetrahydrofuran solution, and add to the above reaction solution after purging with nitrogen to remove oxygen. After the addition was completed, it was raised to room temperature, and the stirring reaction was continued for 5h. After the reaction, the solvent was removed under reduced pressure, and the crude product was separated by column chromatography (eluent: 5 / 2 dichloromethane / petroleum ether) to obtain a red glassy solid with a yield of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com