Human NT-proBNP preparation capable of stable preservation and preparation method thereof
A nt-probnp, stable technology, applied in the field of medicine and biology, can solve the problems of high production conditions, huge investment in eukaryotic expression system, long expression cycle, etc., and achieve the effect of high expression yield, strong stability and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Synthesis, cloning, transformation, and double-enzyme digestion identification of the target gene
[0057] The optimized nucleotide sequence encoding human NT-proBNP as described above was synthesized by Shanghai Genomics Bioengineering Technology Service Co., Ltd. After the nucleotide sequence was synthesized, the sequence was cloned into the pET32a vector.
[0058] Transformation of BL21 (DE3) expression strain: Take out the competent cells from the -80°C refrigerator and place them on ice to thaw. After thawing, add 1μg of the synthesized plasmid, ice bath for 30min, heat shock at 42°C for 60-90s, place on ice for 2min (do not move during this process), add 900ul LB medium, shake at 37°C and 160rpm for 45min, take out 100μl and spread on Ampicillin-resistant LB plates were cultured upside down at 37°C overnight. After the growth of monoclonal colonies, culture the monoclonal colonies and preserve the strains.
Embodiment 2
[0060] Example 2 Optimization of fermentation conditions of BL21 (DE3) strain transformed with NT-proBNP
[0061] Determination of the growth curve of the strain: use 1% and 0.1% glycerol bacteria in the LB medium respectively, and measure the OD at intervals 600 , respectively to make growth curves to determine the appropriate inoculum size. Finally, 0.1% was selected as the optimal inoculum volume, and the seeds were cultured with shaking overnight.
[0062] Determination of carbon source: 0.1% of glycerol bacteria were inoculated into LB medium (Amp100μg / ml), shaken at 37°C and 210rpm overnight. Inoculate 1% of the inoculum into 15ml medium (on the basis of 2YT medium, respectively take two concentrations of sucrose, glucose, and glycerol, two concentrations of 0.5% and 1%, Amp100μg / ml, and culture on a shaking table at 37°C , 220rpm. Cultivate until OD600>0.8 (about 3-4h), add 1mM IPTG to induce. After 4h, collect the bacteria to measure the OD value of the collected bac...
Embodiment 3
[0072] Expression and purification of embodiment three recombinant human NT-proBNP proteins
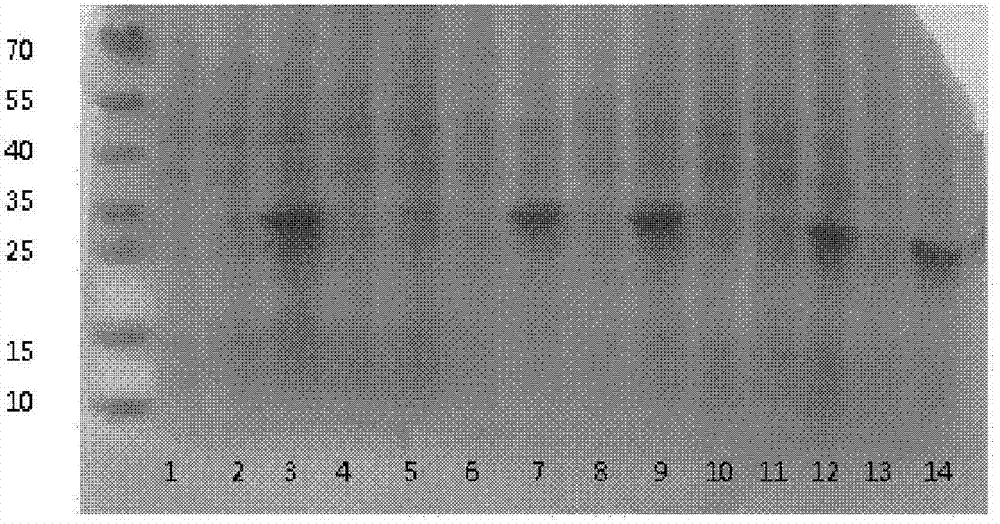
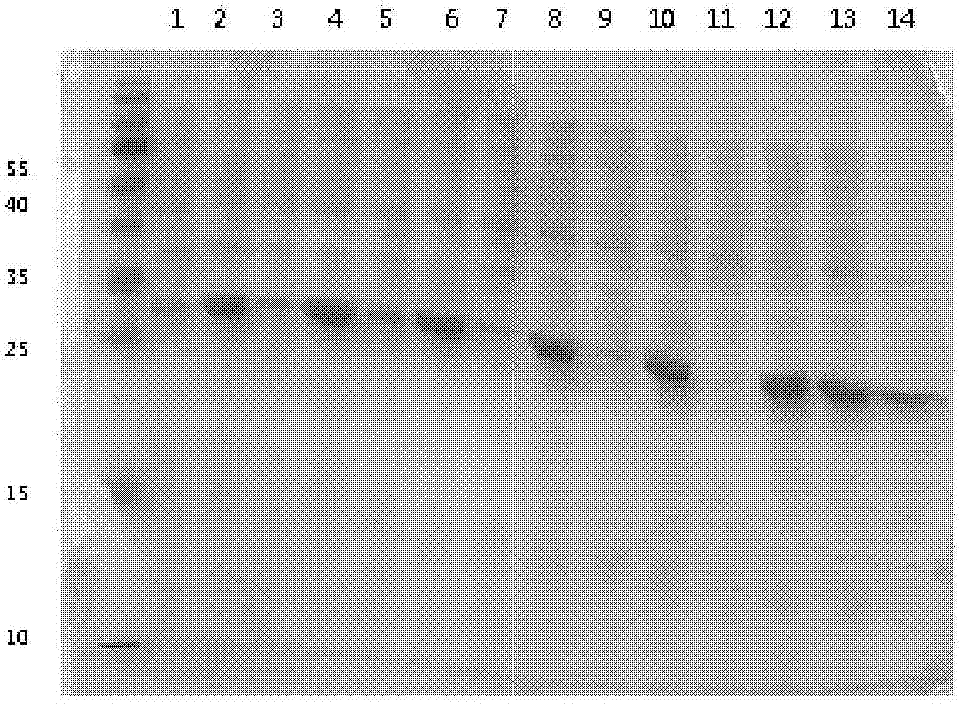
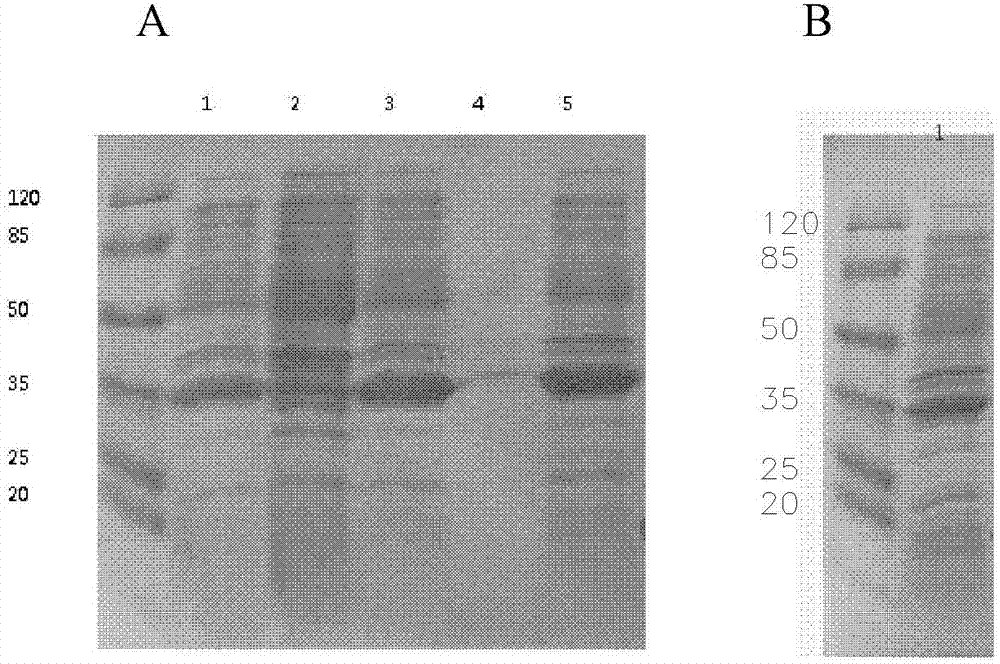
[0073] The expression strain BL21 (DE3) transformed with the vector pET32a containing the human NT-proBNP sequence was cultivated using the optimized culture conditions screened in Example 2. Inoculate with 1% inoculum during the cultivation process, use 1% glycerol as fermentation carbon source, nitrogen source is 0.5% yeast powder, 0.5% peptone, 1% NH 4 Cl, the inorganic salt additive is 5mM magnesium sulfate, and the bacteria collection time is 6 hours. After expression, the strain was homogeneously broken and centrifuged under high pressure. The results of electrophoresis showed that the combination of the strain and the vector achieved efficient and soluble expression of the target protein. The results are as follows: Image 6 shown.
[0074] The loading order of the electrophoresis results is from left to right: supernatant of crushed cells, crushed cells of precipitates, tota...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com