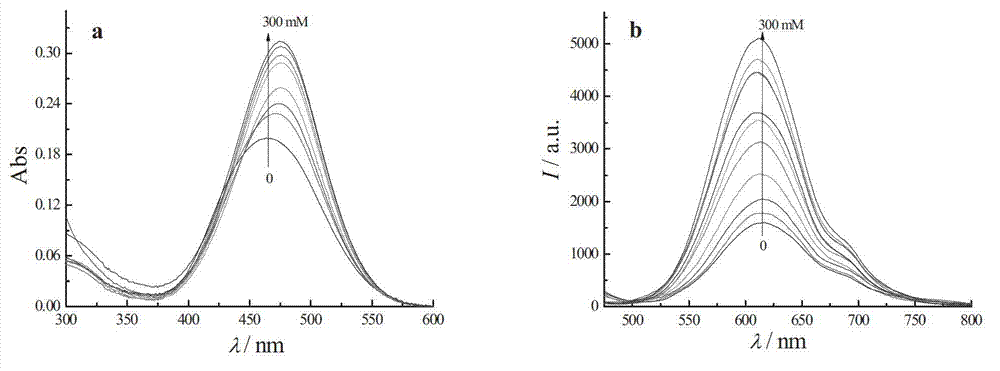
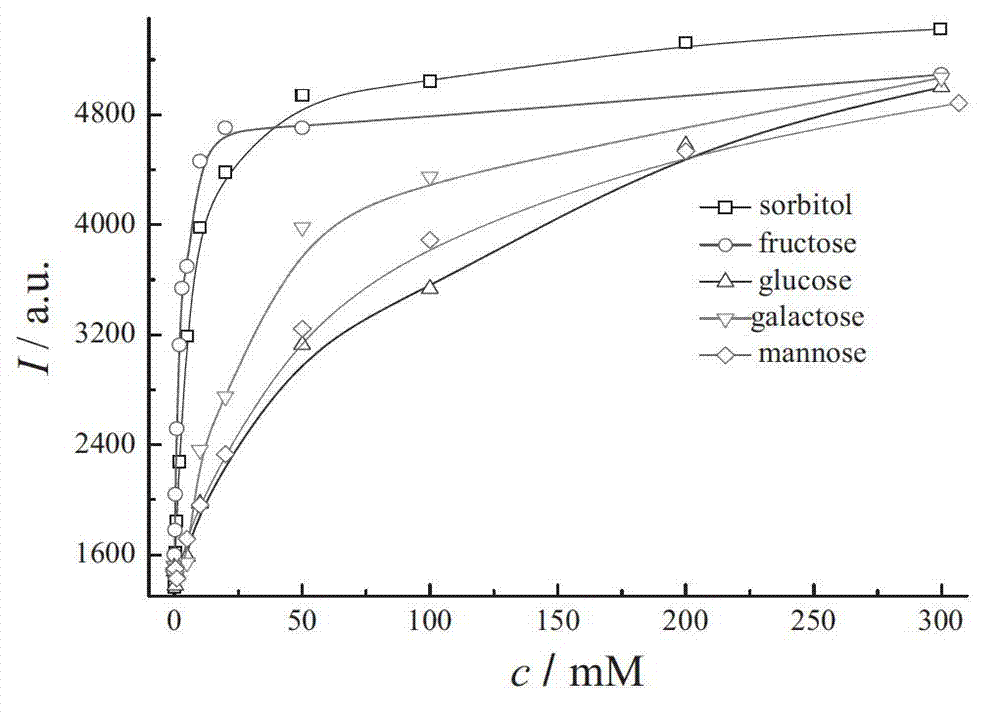
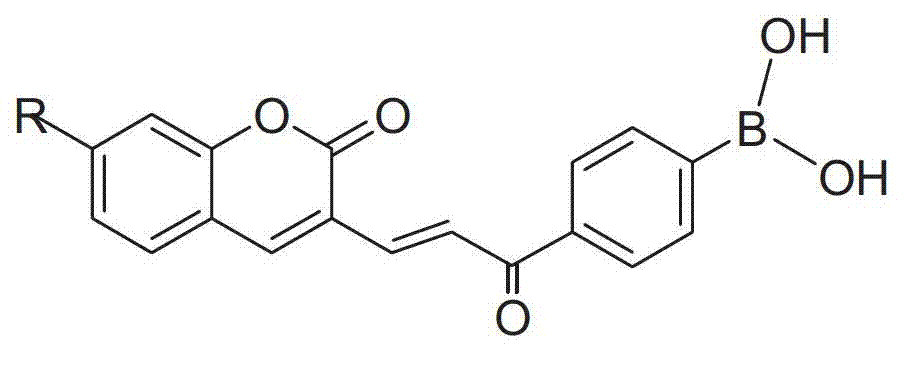
Coumarins compound containing aryl boric acid and application thereof in detection of sugar
An arylboronic acid and coumarin technology, applied in the field of sugar detection, can solve the problems of limited application and short emission wavelength, and achieve the effects of eliminating interference, good water solubility, and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Synthesis of compound CSP
[0030] (1) Synthesis of compound 1
[0031] Add 1.0g 4-bromoacetophenone (5.05mmol), 1.53g bis(pinacolate) diboron (6.03mmol), 1.48g potassium acetate (15.11mmol), 44.11mg [1, 1′-bis(diphenylphosphino)ferrocene]palladium dichloride (0.06mmol) and 15mL of dioxane were heated to reflux, followed by TLC spotting until the disappearance of the raw material. The solvent was removed, purified by silica gel column, and the developing solvent was petroleum ether: ethyl acetate = 50:1 (v / v) to obtain 1.15 g of light yellow viscous product 1 with a yield of 92.5%. 1 H NMR (400MHz, CDCl 3 )δ: 7.84(d, J=8.2Hz, 2H), 7.80(d, J=7.7Hz, 2H), 2.51(s, 3H), 1.26(s, 12H).
[0032] (2) Synthesis of 7-diethylaminocoumarin (compound 2)
[0033] Add 6.0g of 4-diethylamino salicylaldehyde (28.69mmol), 10.84g of diethyl malonate (67.75mmol), 90mL of absolute ethanol and 3mL of piperidine into a 250mL three-necked flask, the solution is dark red, and hea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com