Preparation of supported gold-nickel alloy nanocatalyst
A gold-nickel alloy, supported technology is applied in the field of preparation of gold-nickel alloy nano-catalysts, and achieves the effects of narrow particle size distribution, metal consumption saving, and high catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
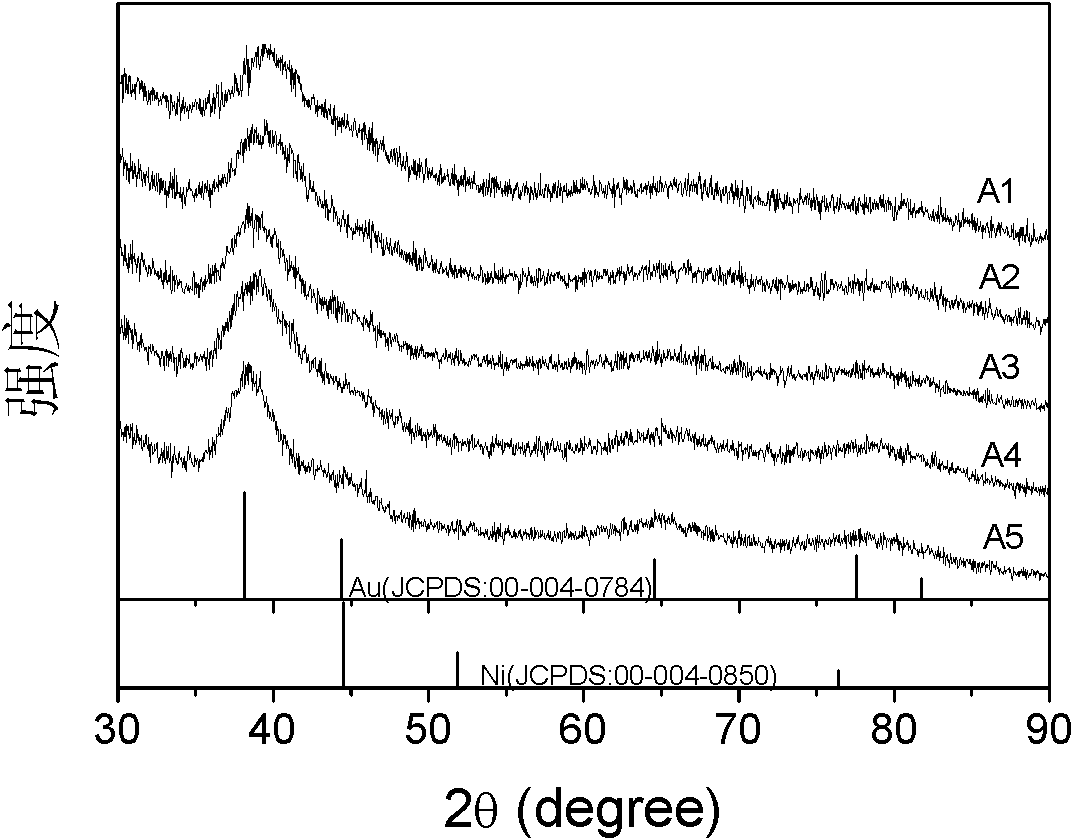
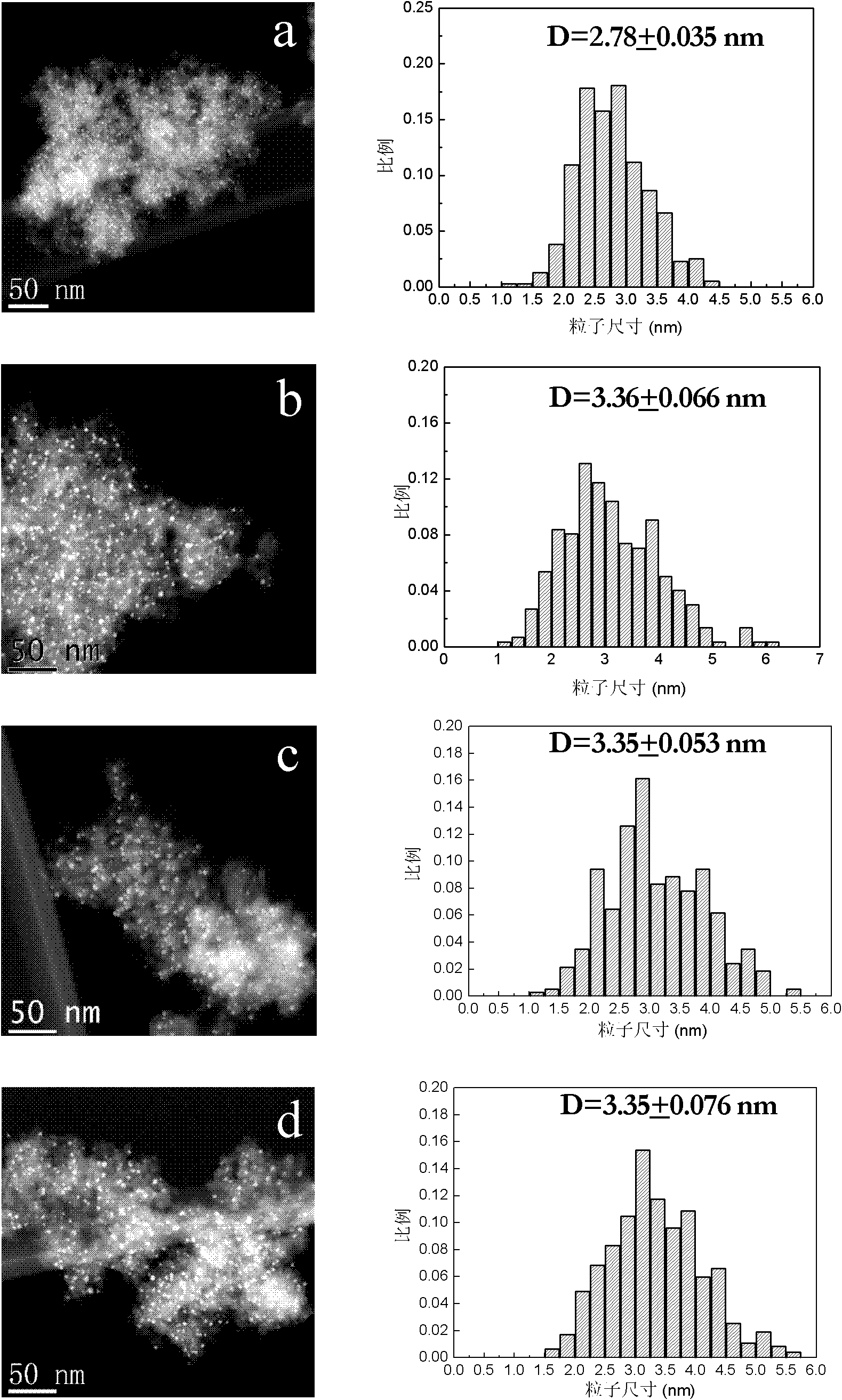
Image
Examples
Embodiment 1
[0038] According to the synthetic method in the literature (Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Journal of the American Chemical Society 1998, 120, 6024-6036), SBA-15 with abundant hydroxyl groups on the surface was prepared. Under the condition of 40℃, dissolve 2g P123 in 15g water and 60g hydrochloric acid (2M) aqueous solution. After it is completely dissolved, under constant stirring, add 4.25g ethyl orthosilicate, continue stirring for 24h, put it into the reaction Crystallized in a kettle at 100°C for 48 hours, filtered, and dried to obtain white powder SBA-15.
[0039] Put 8g of SBA-15 into a dry three-necked flask, dry in an oven at 100°C for 2 hours, add 400mL of absolute ethanol after cooling down to room temperature, stir well, add 21.2mL of 3-aminopropyltriethoxysilane (99 %, Acros Organics) was refluxed at 80°C for 24 hours, and then the solid precipitate was filtered and washed with absolute ethanol until no blue flocculent precipitate was...
Embodiment 2
[0041] 8g SiO 2 Put it into a dry three-necked flask, dry in an oven at 100°C for 2 hours, add 400mL of absolute ethanol after cooling down to room temperature, stir well and add 21.2mL of 3-aminopropyltriethoxysilane (99%, Acros Organics) Reflux at 80°C for 24 hours, then filter the solid precipitate and wash with absolute ethanol until no blue flocculent precipitate is detected with copper nitrate. Then put the solid into an oven at 60°C for 6 hours to get the amino functionalized carrier SiO 2 -APTES.
Embodiment 3
[0043] Add 16 mL of deionized water and 6 mL of chloroauric acid aqueous solution containing 9.56 mg / mL gold to a 100 mL beaker, stir well at room temperature, and then add 1.1118 g SiO 2 -After APTES continues to stir for 30 minutes, it is filtered and washed with 1000 mL of deionized water. The obtained solid was re-dispersed in a 100 mL beaker containing 11 mL of deionized water, and 11 mL of a 0.2M sodium borohydride aqueous solution was added dropwise while stirring at room temperature. After stirring for 15 minutes, it was filtered and washed with 1000 mL of deionized water. The obtained solid was dispersed into 11mL deionized water and 0.2540g Ni(NO 3 ) 2 ·6H 2 In the beaker of O, add 30mL of 0.2M tert-butylammonium borane aqueous solution dropwise while stirring at room temperature, continue stirring for 15 minutes, filter and wash with 1000mL deionized water, dry at room temperature and put it in an oven at 80°C for 12 hours. The catalyst obtained at this time is denote...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com