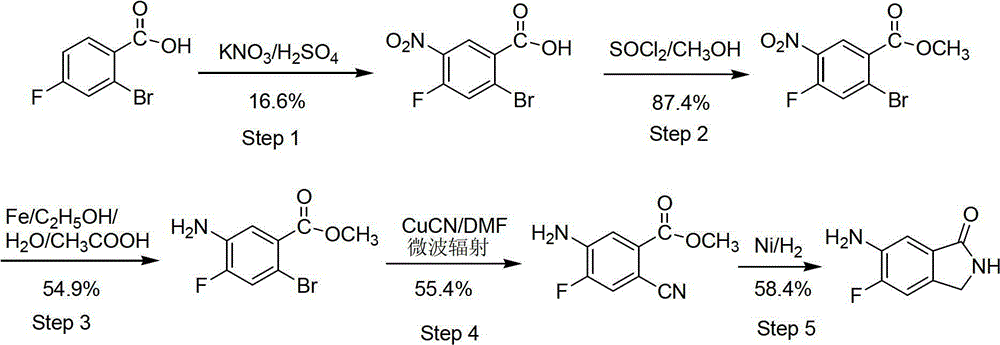
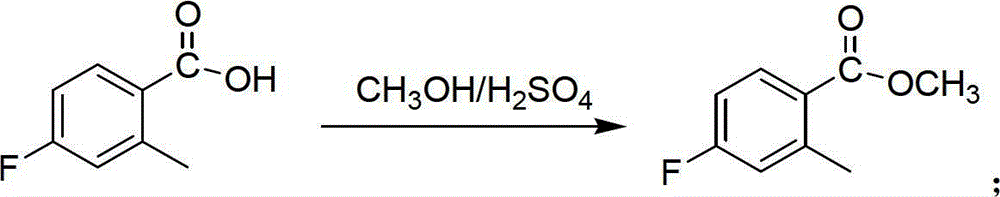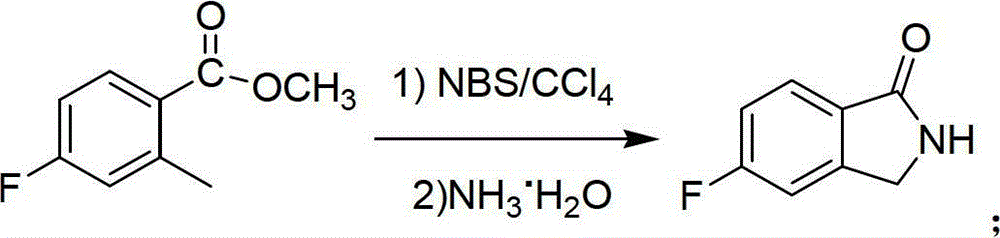Preparation method of 6-amino-5-fluorine-1-isoindolinone
A technology of isoindolinone and amino group is applied in the field of preparation of indole derivatives, which can solve the problems of harsh reaction conditions, low yield, high toxicity and the like, and achieves the effects of suitable reaction conditions, cost reduction and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A preparation method of 6-amino-5-fluoro-1-isoindolinone, comprising the steps of:
[0023] 1) In a 100mL three-neck flask equipped with a magnetic stirrer, slowly drop 5.0mL of concentrated sulfuric acid into 25mL of methanol dissolved in 5.0g of 4-fluoro-2-methylbenzoic acid, heat to boil and reflux for 5h, stop the reaction, Spin off the solvent under reduced pressure to obtain a solid, dissolve the obtained solid with 100 mL of ethyl acetate to obtain an ethyl acetate solution, wash the ethyl acetate solution with an equal volume of water, saturated sodium bicarbonate solution and saturated brine successively, and dry over anhydrous sodium sulfate After washing the ethyl acetate solution, spin off the solvent to obtain 4.94g of 4-fluoro-2-methylbenzoic acid, the yield of this step is 90.6%; the obtained substance is characterized by mass spectrometry EI-MS (m / z) detection The data is 169.4[M+1] + , indicating that the obtained substance is 4-fluoro-2-methylbenzoic ...
Embodiment 2
[0028] 1) In a 1000mL three-neck flask equipped with a magnetic stirrer, slowly drop 60mL of concentrated sulfuric acid into 600mL of methanol dissolved in 100g of 4-fluoro-2-methylbenzoic acid, heat to boil and reflux for 12h. Stop the reaction, spin off the solvent under reduced pressure, dilute and dissolve with 800mL ethyl acetate, wash with an equal volume of water, saturated sodium bicarbonate solution and saturated brine successively, dry over anhydrous sodium sulfate, and spin off the solvent to obtain 4-fluoro-2 -Toluic acid 100.1g, the yield of this step is 91.8%; the obtained characterization data of the obtained substance detected by mass spectrometry EI-MS (m / z) is 168.9[M+1] + , indicating that the obtained substance is 4-fluoro-2-methylbenzoic acid;
[0029]2) In a 1000mL three-neck flask equipped with a magnetic stirrer, add 120.0g of N-bromosuccinimide and 4.0g of benzoyl peroxide in sequence to dissolve 80.0g of 4-fluoro-2-methylbenzoic acid Methyl ester in ...
Embodiment 3
[0033] 1) In a 5000mL three-necked flask equipped with a magnetic stirrer, slowly drop 300.0mL of concentrated sulfuric acid into 3500mL of methanol dissolved in 300.0g of 4-fluoro-2-methylbenzoic acid, heat and boil under reflux for 15 hours, and stop the reaction , spin off the solvent under reduced pressure to obtain a solid, dissolve the resulting solid with 2000mL ethyl acetate to obtain an ethyl acetate solution, wash the ethyl acetate solution with an equal volume of water, saturated sodium bicarbonate solution and saturated brine successively, and wash the ethyl acetate solution with anhydrous sodium sulfate Dry and wash the ethyl acetate solution, spin off the solvent to obtain 292.8 g of 4-fluoro-2-methylbenzoic acid, the yield of this step is 89.5%; the obtained substance is detected by mass spectrometry EI-MS (m / z) The characterization data is 169.2[M+1] + , indicating that the obtained substance is 4-fluoro-2-methylbenzoic acid;
[0034] 2) In a 5000mL three-neck...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com