Method for preparing atazanavir
A technology of phenyl and organic solvents, which is applied in the field of preparation of atazanavir, can solve the problems of unsuitability for industrial production, complex separation and purification of products, and achieve the effects of less pollutants, low equipment and operation requirements, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
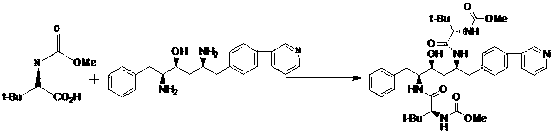
[0034] Example 1: Preparation of 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxy-5(S)-2,5-diamino-6-phenyl-2-azahexane
[0035] In a clean reaction flask, add 11.25g (20mmol) of 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxyl-5(S)-2,5-bis[( tert-butoxycarbonyl)amino]-6-phenyl-2-azahexane and 45ml of dichloromethane, add 9.3g of hydrochloric acid dropwise; after dropping, raise the temperature to 40~45°C for about 3h; TLC analysis and tracking, After the reaction is complete, cool down to 25°C, add 13.2g of N-methylmorpholine, stir at this temperature for 1h, separate layers, dry the organic layer with anhydrous sodium sulfate for 1 hour, filter, rinse the filter cake with a small amount of solvent, and depressurize Concentration to dryness afforded 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxy-5(S)-2,5-diamino-6-phenyl-2-nitrogen as a solid Heterohexane 6.87g, yield 95.2%.
Embodiment 2
[0036] Example 2: Preparation of atazanavir monomer
[0037] In a clean reaction flask, add 6.87g of 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxyl-5(S)-2,5-diamino-6-benzene Base-2-azahexane, 50ml of dichloromethane and 3.83g of N-methylmorpholine, then added 4.16g (22mmol) of N-methoxycarbonyl-L-tert-leucine and 7.2g (24mmol ) of DEPBT, stirred at 35°C for 2h, followed by TLC analysis until the end of the reaction. Add 40ml of 2% sodium hydroxide dropwise, stir for 0.5h, let stand to separate layers, wash the organic layer with 2*40ml of water, remove dichloromethane under reduced pressure, add 2*40ml of ethanol, concentrate to dryness under reduced pressure; in the residual liquid Add 45ml of ethanol and 55ml of water, heat to 60°C, slowly cool down to about 0°C, stir for 3 hours, filter with suction, wash the filter cake with 0°C ethanol and water (1:2), and wash the mixture twice (40ml) , drained, and dried to obtain 11.28 g of solid atazanavir monomer. Yield: 84.1%
Embodiment 3
[0038] Example 3:Preparation of atazanavir sulfate
[0039] At room temperature of 25°C, dissolve 70.5g of atazanavir monomer in 700g of acetone, slowly add 5M 40.4g of concentrated sulfuric acid dropwise under stirring, and keep warm at this temperature for 2h after the dropping; after the heat preservation is over, cool down To 0 ~ 5 ℃, stirring for 2h. After suction filtration, the filter cake was washed twice with 150ml of acetone, and dried to obtain 76.3g of the product atazanavir sulfate, with a yield of 95.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com