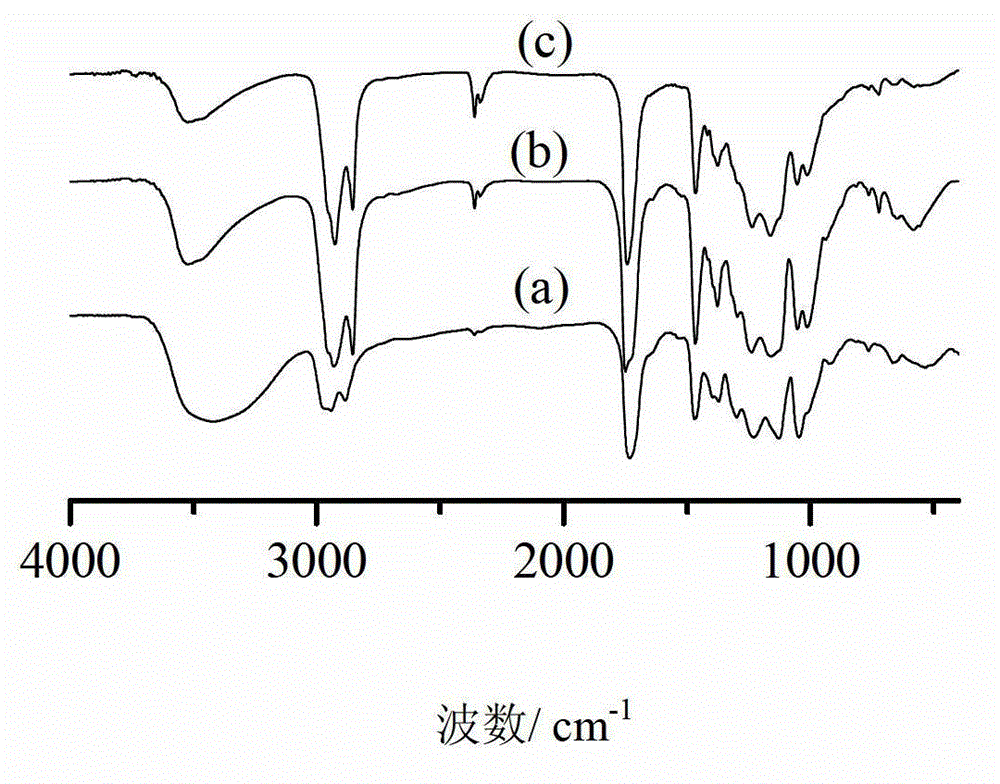
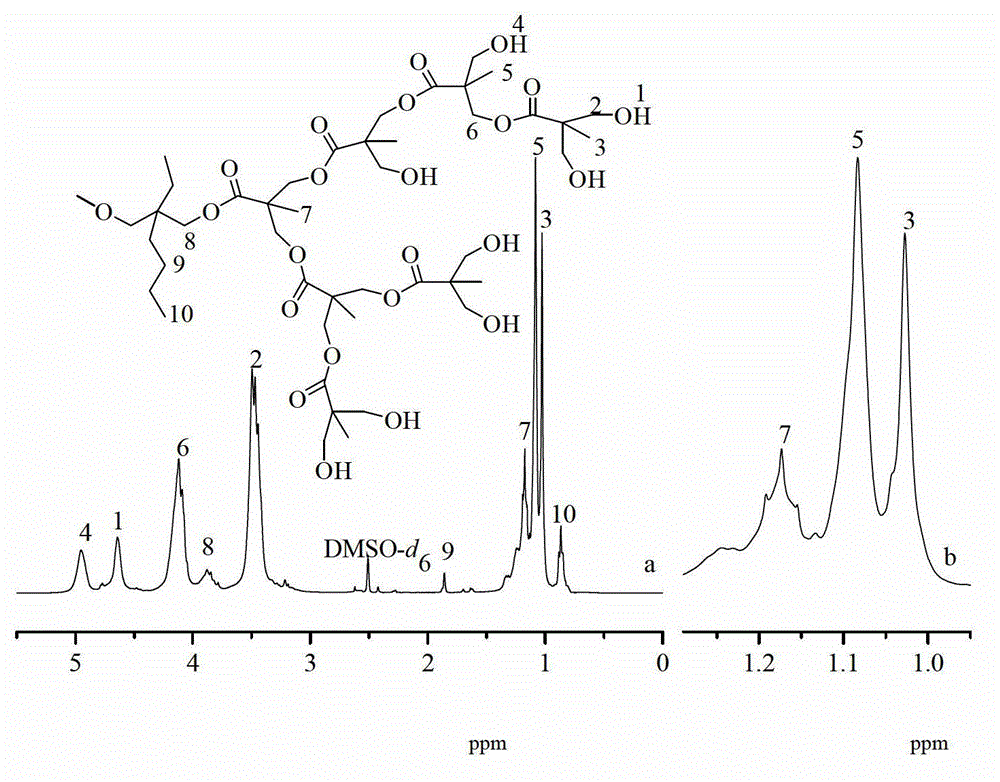
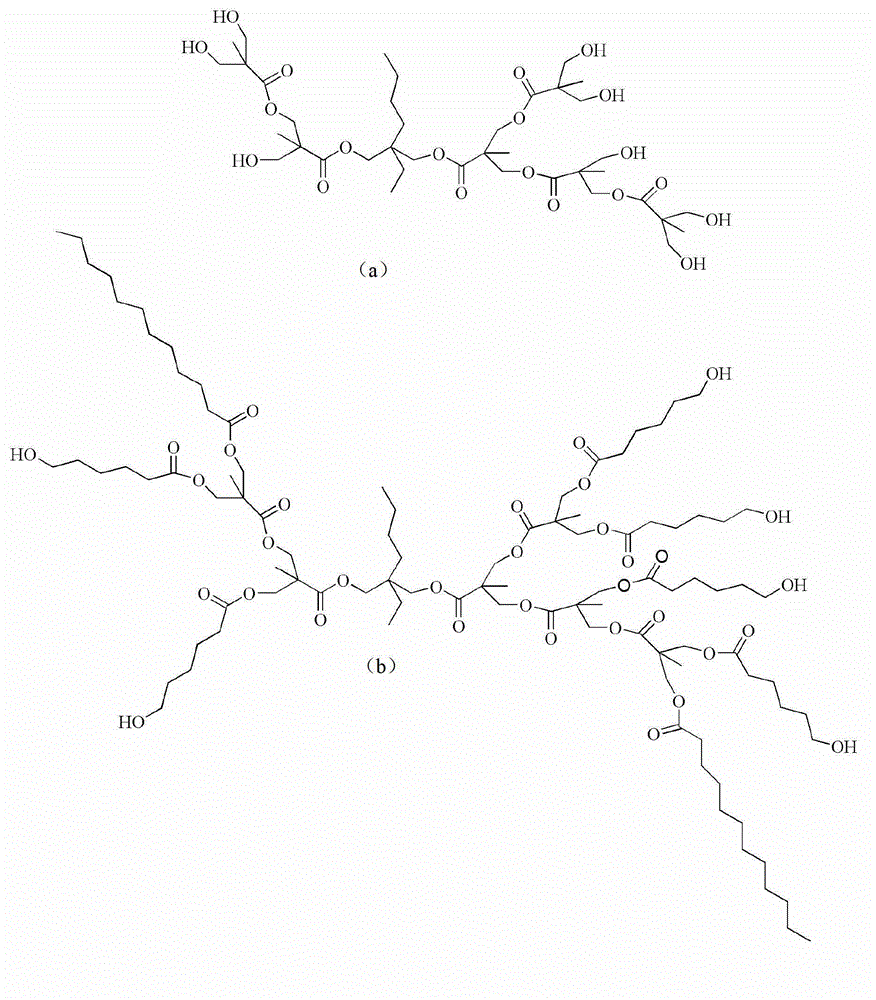
Caprolactone modified hyperbranched polyester and preparation method and application thereof
A technology for modifying hyperbranched polyester and caprolactone, which is applied in the direction of polyester coatings and coatings, can solve the problems of incompatibility with solvents, achieve reduced viscosity, increased activity, and good coating performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1. Raw material preparation
[0062] Polyol: Ethylbutylpropylene glycol 16g (0.1mol); Dimethylolalkyl carboxylic acid: Dimethylol propionic acid 80.4g (0.6mol); Fatty acid: Lauric acid 40g (0.2mol); Caprolactone: 68.4g (0.6mol); acidic catalyst: 0.4g of p-toluenesulfonic acid; organotin catalyst: 0.05g of dibutyltin dilaurate; reflux solvent: 11g of xylene.
[0063] 2. Preparation
[0064] (a) When equipped with agitator, thermometer, water cooling condensate separator, N 2 Add 16g of ethyl butyl propylene glycol, 26.8g of dimethylol alkyl carboxylic acid (dimethylol propionic acid), 0.1g of acid catalyst p-toluenesulfonic acid and 2.5g of reflux solvent xylene into the inlet four-necked flask , at 140°C N 2 React under protection until no water distills out, then gradually rise to 180°C to react until the acid value is lower than 20mgKOH / g (according to GB / T 12008.5-89 to determine the polymer acid value); continue to add 53.6g of dimethylol alkyl carboxyl Acid, 0....
Embodiment 2
[0079] 1. Raw material preparation
[0080]Polyol: Neopentyl Glycol 10.4g (0.1mol); Dimethylolalkyl Carboxylic Acid: Dimethylolpropionic Acid 80.4g (0.6mol); Fatty Acid: Lauric Acid 40g (0.2mol); Caprolactone: 68.4g (0.6mol); acidic catalyst: concentrated sulfuric acid 0.7g; organotin catalyst: 0.05g dibutyltin dilaurate; reflux solvent: toluene 15g
[0081] 2. Preparation
[0082] (a) When equipped with agitator, thermometer, water cooling condensate separator, N 2 In the inlet four-necked flask, add 10.4g of neopentyl glycol, 26.8g of dimethylol alkyl carboxylic acid, 0.15g of acid catalyst concentrated sulfuric acid and 3g of toluene. 2 React under protection until no water distills off, then gradually rise to 180°C to react until the acid value is lower than 20mgKOH / g; continue to add 53.6g of dimethylolalkyl carboxylic acid, 0.3g of acid catalyst concentrated sulfuric acid and 6g of reflux solvent, at 140°CN 2 React under protection until no water distills off, then g...
Embodiment 3
[0091] 1. Raw material preparation
[0092] Polyol: YmerN120 (molecular weight 1000) 70g (0.07mol); Dimethylolalkyl carboxylic acid: dimethylolbutyric acid 62.16g (0.42mol); Fatty acid: oleic acid 56.4g (0.2mol); Caprolactone : 47.88g (0.42mol); acidic catalyst: 0.4g of hypophosphorous acid; organic tin catalyst: 0.035g of dibutyltin dilaurate; reflux solvent: 15g of xylene.
[0093] 2. Preparation
[0094] (a) When equipped with agitator, thermometer, water cooling condensate separator, N 2 Into the inlet four-necked flask, add 70g of polyol Ymer N120, 20.72g of dimethylol butyric acid, 0.1 acid catalyst hypophosphorous acid and 3g of xylene, at 140°C N 2 React under protection until no water distills out, then gradually rise to 180°C to react until the acid value is lower than 20mgKOH / g; continue to add 41.44g dimethylol butyric acid, 0.2g hypophosphorous acid and 6g reflux solvent xylene, at 140°C N 2 React under protection until no water distills out, and then graduall...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| gloss | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com