Electrochemical reduction CO2 electrolytic tank using bipolar membrane as diaphragm and application of electrochemical reduction CO2 electrolytic tank
A bipolar membrane and electrochemical technology, applied in the direction of diaphragm, electrolysis process, electrolysis components, etc., can solve the problems of high electrolysis voltage and large energy loss, and achieve the effect of reducing electrolysis voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
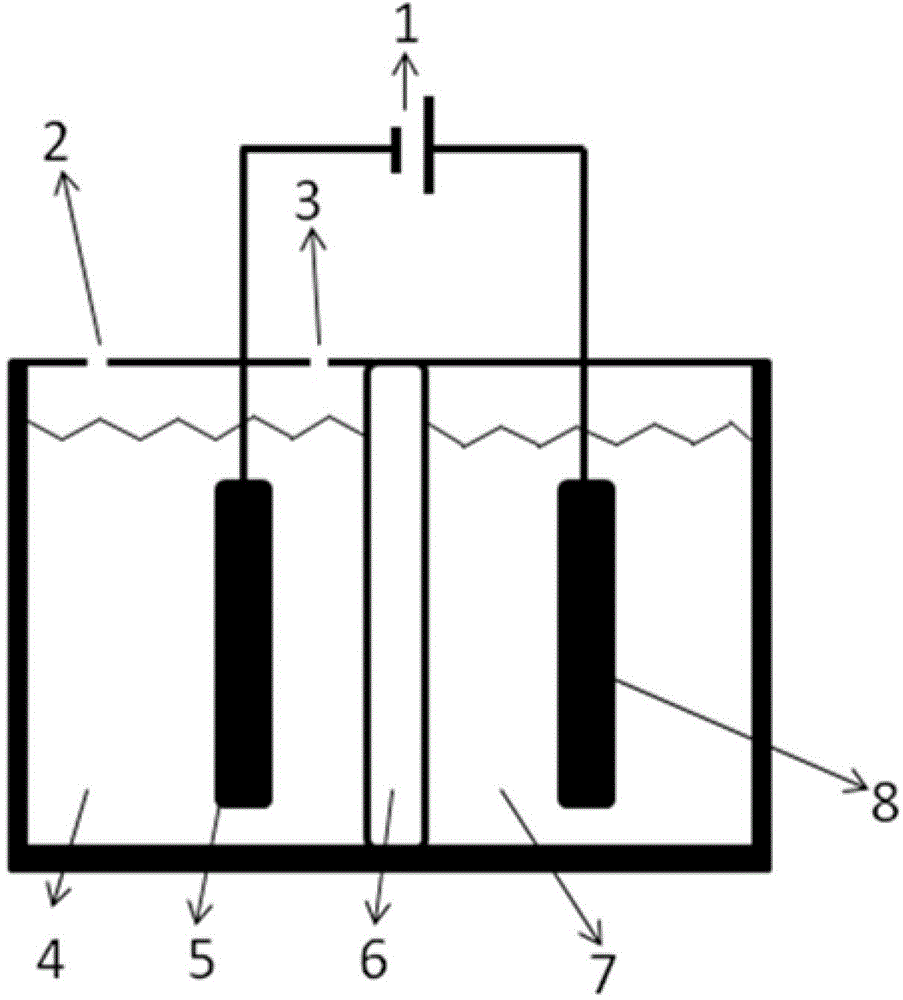
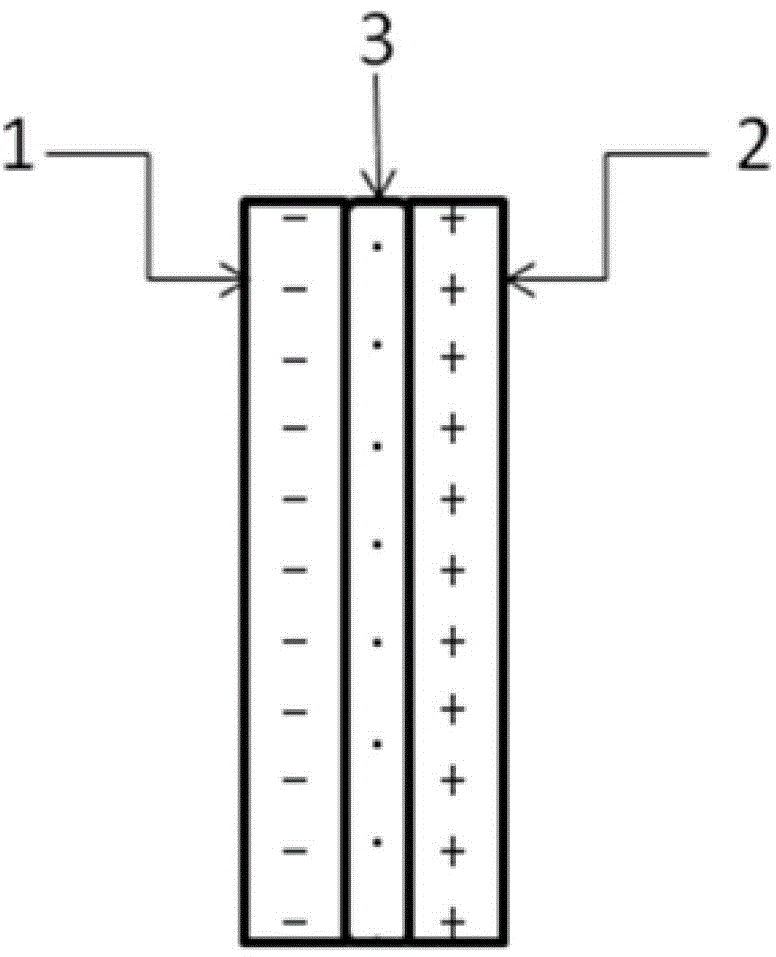
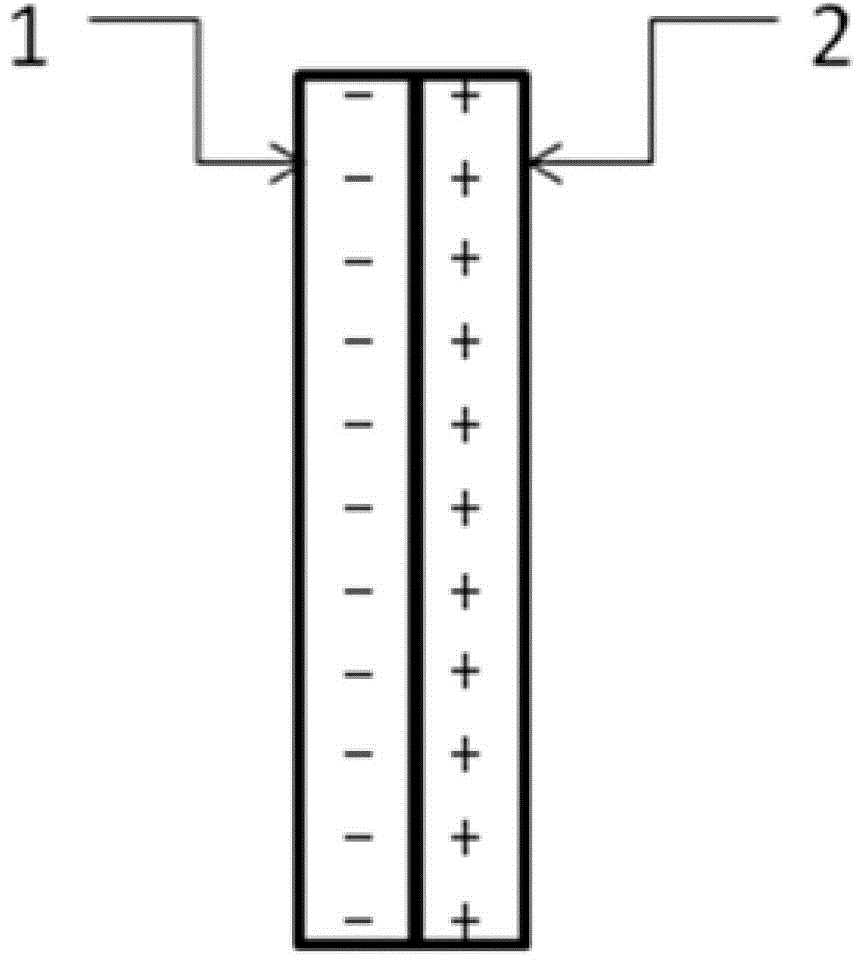
[0052] like figure 1 As shown, under room temperature conditions, a bipolar membrane is used to separate the electrolytic cell into a cathode chamber and an anode chamber, wherein the catholyte is a mixed aqueous solution containing 0.2 mol / L potassium bicarbonate and 0.5 mol / L potassium hydroxide, and the anolyte The concentration of potassium iodide in the medium is 1 mol / liter, and the concentration of sulfuric acid is 0.5 mol / liter; lead sheet is used as cathode and platinum sheet is used as anode respectively, and the dimensions of lead sheet and platinum sheet are both: 1 cm (length) × 1 cm (width). Pass CO2 through the gas port to the anode electrolysis chamber for 30 minutes 2 Gas, control the flow rate of carbon dioxide gas to be 150 ml / min. Turn on the electrolysis power supply, and control the current density of the constant current electrolysis to 25A / m 2 、125A / m 2 、200A / m 2 、250A / m 2 , electrolyzed for 2 hours, carbon dioxide gets electrons on the cathode Pb...
Embodiment 2
[0057]At room temperature, a bipolar membrane is used to separate the electrolytic cell into a cathode chamber and an anode chamber, wherein the catholyte is a mixed solution containing 0.2 mol / L potassium bicarbonate and 0.5 mol / L potassium hydroxide, and potassium iodide in the anolyte The concentration of sulfuric acid is 1 mol / L, and the concentration of sulfuric acid is 0.5 mol / L; copper sheets are used as cathodes and platinum sheets are used as anodes respectively, and the dimensions of copper sheets and platinum sheets are both: 1 cm (length) × 1 cm (width ). Pass CO2 through the gas port to the anode electrolysis chamber for 30 minutes 2 Gas, control the flow rate of carbon dioxide gas to be 150 ml / min. Turn on the electrolysis power supply, and control the current density of constant current electrolysis to 25A / m 2 , electrolyzed for 1 hour, carbon dioxide gets electrons on the cathode Cu sheet to undergo an electrochemical reduction reaction, and iodide ions under...
Embodiment 3
[0061] At room temperature, a bipolar membrane is used to separate the electrolytic cell into a cathode chamber and an anode chamber, wherein the catholyte is a mixed solution containing 0.2 mol / L potassium bicarbonate and 0.5 mol / L potassium hydroxide, and potassium iodide in the anolyte The concentration of sulfuric acid is 2 mol / liter, and the concentration of sulfuric acid is 0.5 mol / liter; silver wire is used as cathode and platinum sheet is used as anode respectively, and the size of platinum sheet is 1 cm (length) × 1 cm (width). Pass CO2 through the gas port to the anode electrolysis chamber for 30 minutes 2 Gas, control the flow rate of carbon dioxide gas to be 150 ml / min. Turn on the electrolysis power supply, and control the current density of constant current electrolysis to 25A / m 2 , electrolyzed for 1 hour, carbon dioxide gets electrons on the cathode Ag electrode to undergo an electrochemical reduction reaction, and iodide ions undergo an oxidation reaction on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com