Method for constructing protein three-dimensional structure based on specific cross-linked tyrosine
A three-dimensional structure, protein technology, applied in the field of cross-linking mass spectrometry, can solve the problems of difficult functionalization, no reports of tyrosine residue cross-linking technology, etc., and achieve the effect of reducing the search scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
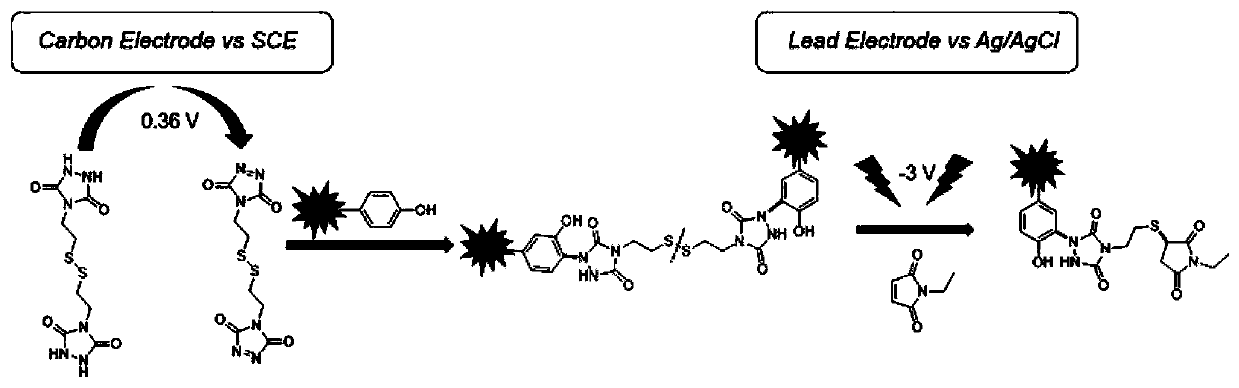
[0024] This example discloses a method for preparing a specific cross-linking tyrosine cross-linking agent, which includes three steps:
[0025]
[0026] The first step: Synthesis of 1,2,2-dicarboxylic acid ethylphenylhydrazine (EPHD, 1)
[0027] 0.5 g of diphenyl carbonate (1 eq., 2.3 mM) was melted in a 100 mL flask at 80° C., and then 0.5 g of ethyl carbamate (2 eq., 4.6 mM) was added to react for 1 h. Purification by silica gel column chromatography (EtOAc / petroleum ether solution 1:3) afforded 1 as a white solid. 1 H NMR (400MHz, DMSO) δ9.68(s, 1H), 9.25(s, 1H), 7.41(t, J=7.9Hz, 2H), 7.24(t, J=7.4Hz, 1H), 7.11(d , J=7.9Hz, 2H), 4.07(dd, J=14.1, 7.1Hz, 2H), 1.19(t, J=7.1Hz, 3H). 13 C NMR (101MHz, DMSO) δ156.90, 155.32, 151.07, 129.95, 125.86, 121.94, 61.17, 14.98.
[0028] The second step: the synthesis of compound 2
[0029] 0.226 g of ethylphenylhydrazine-1,2-dicarboxylate (Compound 1, 1 eq.) was heated to 80°C under an inert atmosphere. A mixture of triethylamine ...
Embodiment 2
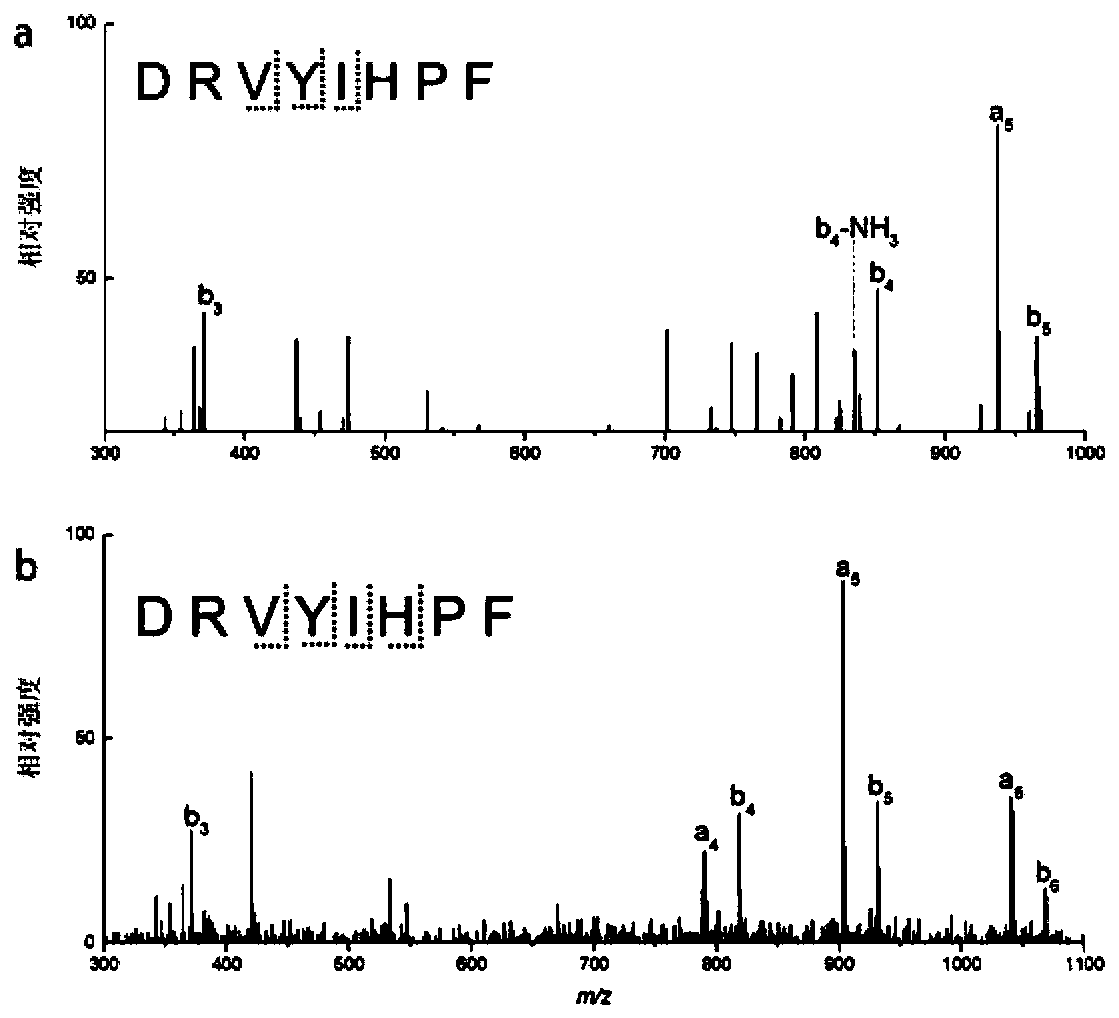
[0033] Crosslinker specifically targets tyrosine in angiotensin Ⅱ
[0034] (1) Chemical cross-linking reaction: Angiotensin II was dissolved in 100 mM PB buffer at pH 7.40. 3 mL of 1 mM insulin and the cross-linking agent were reacted at a voltage of 0.36 V for 4 hours at room temperature at a molar ratio of 1:2.
[0035] (2) Angiotensin II after cross-linking was detected by Agilent 1290 Infinity liquid chromatography-BrukermicrOTOF-QII mass spectrometry (LC-MS n ) for analysis. Before mass spectrometry analysis, Agilent Zorbax300SB-C18 reverse-phase column (4.6×250 mm, 5 μm, column temperature 40° C.) was used for liquid phase separation. The flow rate is 1mL / min; the linear gradient is: 5% B for 0-5min, 5-60% B for 6-55min, 60-98% B for 56-60min, and the mobile phase buffers A and B contain 0.1% B respectively Formic acid in water and acetonitrile. MS 2 Spectra were generated by collision-induced dissociation (CID) at an energy of 15 eV.
[0036] (4) The cross-linked ...
Embodiment 3
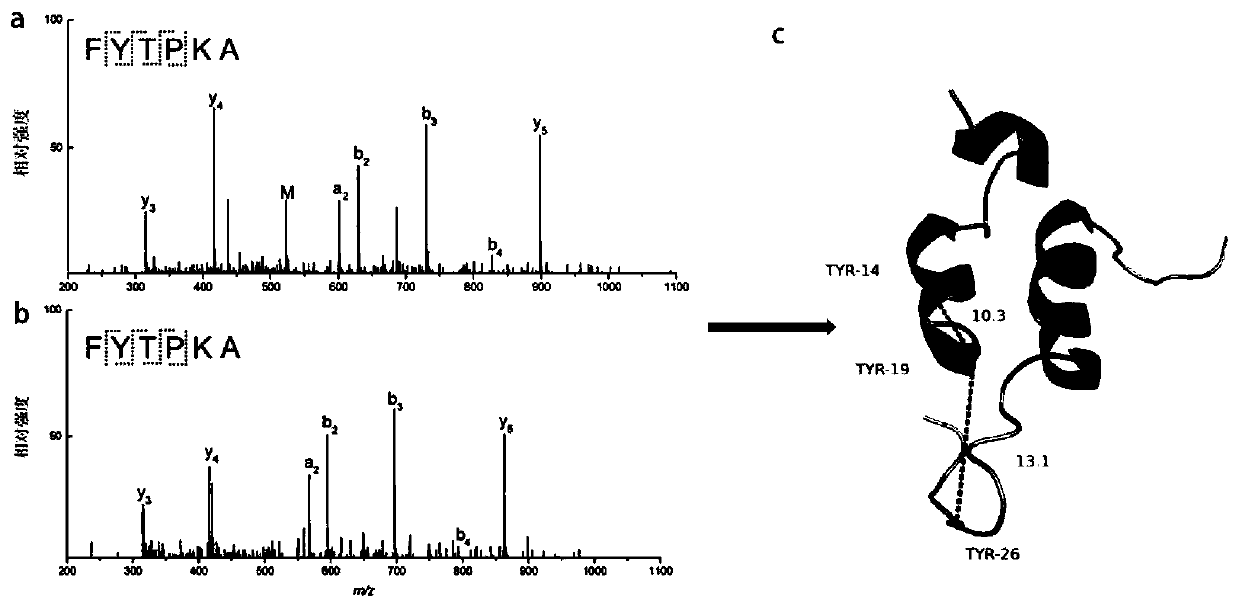
[0041] Three-dimensional structure identification of insulin
[0042] (1) Chemical cross-linking reaction: Insulin was dissolved in 100 mM PB buffer at pH 7.40. 3 mL of 0.2 mM insulin and cross-linking agent were reacted at a voltage of 0.36 V for 4 hours at room temperature at a molar ratio of 1:2, 1:10 and 1:20.
[0043] (2) Use pepsin dissolved in 1% acetic acid solution to carry out enzymolysis reaction on the above solution, incubate at 37°C for 6.5h, the mass ratio of pepsin to protein is 50:1, and the final concentration of insulin after enzymolysis is 0.5mg / mL.
[0044] (3) Agilent 1290 Infinity liquid chromatography-Bruker micrOTOF-QII mass spectrometry (LC-MS n ) for analysis. Before mass spectrometry analysis, Agilent Zorbax 300SB-C18 reverse phase column (4.6×250 mm, 5 μm, column temperature 40° C.) was used for liquid phase separation. The flow rate is 1mL / min; the linear gradient is: 5% B for 0-5min, 5-60% B for 6-55min, 60-98% B for 56-60min, and the mobile...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com