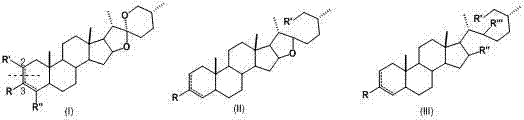
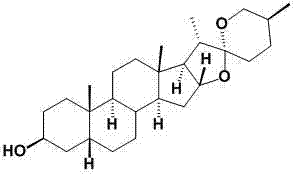
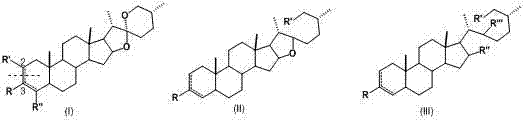
Smilax sapogenin derivative and preparation and applications thereof
A technology of smilagenin and derivatives, which can be used in the field of medicine and can solve problems such as liver toxicity and gastrointestinal reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of Epismilagenin
[0038] (1) Add smilagenin (4.16g, 10mmol), benzoic acid (2.44g, 20mmol), Ph 3 P (5.24g, 20mmol), 50ml of dry THF, DIAD (4.04g, 20mmol) dissolved in 20ml of dry THF was added dropwise with stirring at room temperature, and the reaction was completed at room temperature for 24h. After the reaction was completed, evaporate to dryness under reduced pressure, add water, stir, filter with suction, put on a silica gel column, wash away impurities with petroleum ether, and wash with petroleum ether: ethyl acetate (80:1) to obtain an intermediate product, the structure identification data is as follows:
[0039] m / z: [M+H] + =521.3620, 1 H-NMR (300MHz, CDCl 3 ): δ 8.05(2H, m, H-2',6'), 7.54 (1H, m, H-4'), 7.43 (2H, m, H-3',5'), 4.98 (1H, m , H-3), 4.42 (1H, m, H-26), 3.96 (1H, dd, J=2.52 Hz, J=11.04 Hz, H-26), 3.31 (1H, d, J=11.04 Hz H- 16), 1.08 (3H, d, J=7.11Hz, CH 3 -21), 1.00 (3H, d, J=6.72 Hz, CH 3 -27), 0.98 (3H, s,...
Embodiment 2
[0041] Example 2 Amination of the 3-position of timogenin
[0042]
[0043] (1) Add 0.8g (0.0045mol) NBS, 5ml dichloromethane (or THF) into a 50ml eggplant-shaped bottle, add dropwise triphenylphosphine dissolved in 5ml dichloromethane (or THF) at room temperature under nitrogen protection, After the dropwise addition, stir at 0-30°C for 0.5h, then add 1.2g (0.003mol) of smilagenin dissolved in 10ml of dichloromethane (or THF) dropwise and stir at 10-30°C until the reaction is complete. Add saturation The brine was extracted with dichloromethane (×2), and the organic layer was concentrated under reduced pressure to obtain a solid, which was applied to a silica gel column and washed with petroleum ether: ethyl acetate 100:1-50:1 to obtain a white solid (1.1 g, yield 78 %)[M+H] + =479.25.
[0044] (2) Add 0.96g (0.002mol) of white solid, 10ml DMF (or acetonitrile, acetone, etc.) and 1.62g dimethylamine hydrochloride to a 50ml eggplant-shaped bottle, and react at reflux at 6...
Embodiment 3
[0045] Example 3 Amination of the 3-position of timogenin
[0046]
[0047] Add 0.96g A (0.002mol), 10ml DMF (or acetonitrile, acetone, etc.), 1.7g hexahydropyridine into a 50ml eggplant-shaped flask, and react at reflux at 60°C for 12h. Add water and filter with suction to obtain a white solid, silica gel column, petroleum ether: ethyl acetate (20:1) plus triethylamine to wash the product, and obtain a white solid C (0.38g, yield about 40%) [M+H] + =484.4151, 1 H-NMR (600MHz, CDCl 3 )δ4.4 0(1H,m,J=7.5Hz,J=14.7Hz,H-26),3.96(1H,dd,J=2.58Hz,10.98Hz,H-26),3.30(1H,d, J=10.92Hz,H-16),2.34(4H,br.s,N-2CH 2 ),2.17(1H,br.s,H-3),1.08(3H,d,J=7.08Hz,CH 3 -21),0.99(3H,d,J=6.9Hz,CH 3 -27),0.95(3H,s,CH 3 -19),0.76(3H,s,CH 3 -18).
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com