Amphiphilic type rare earth polyacid catalytic material and preparation method and application thereof
A rare earth polyacid and catalytic material technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problems of low product quality and poor technical level, etc. Achieve the effect of improving solubility, solving solidification and catalyst recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] a. Add 26mmol Na 2 WO 4 2H 2 O was dissolved in 20ml of water, and 2.5mmol LaCl dissolved in it was added dropwise 3 ·7H 2 In the hot aqueous solution of O, adjust the pH=7.0-7.5 with acetic acid.
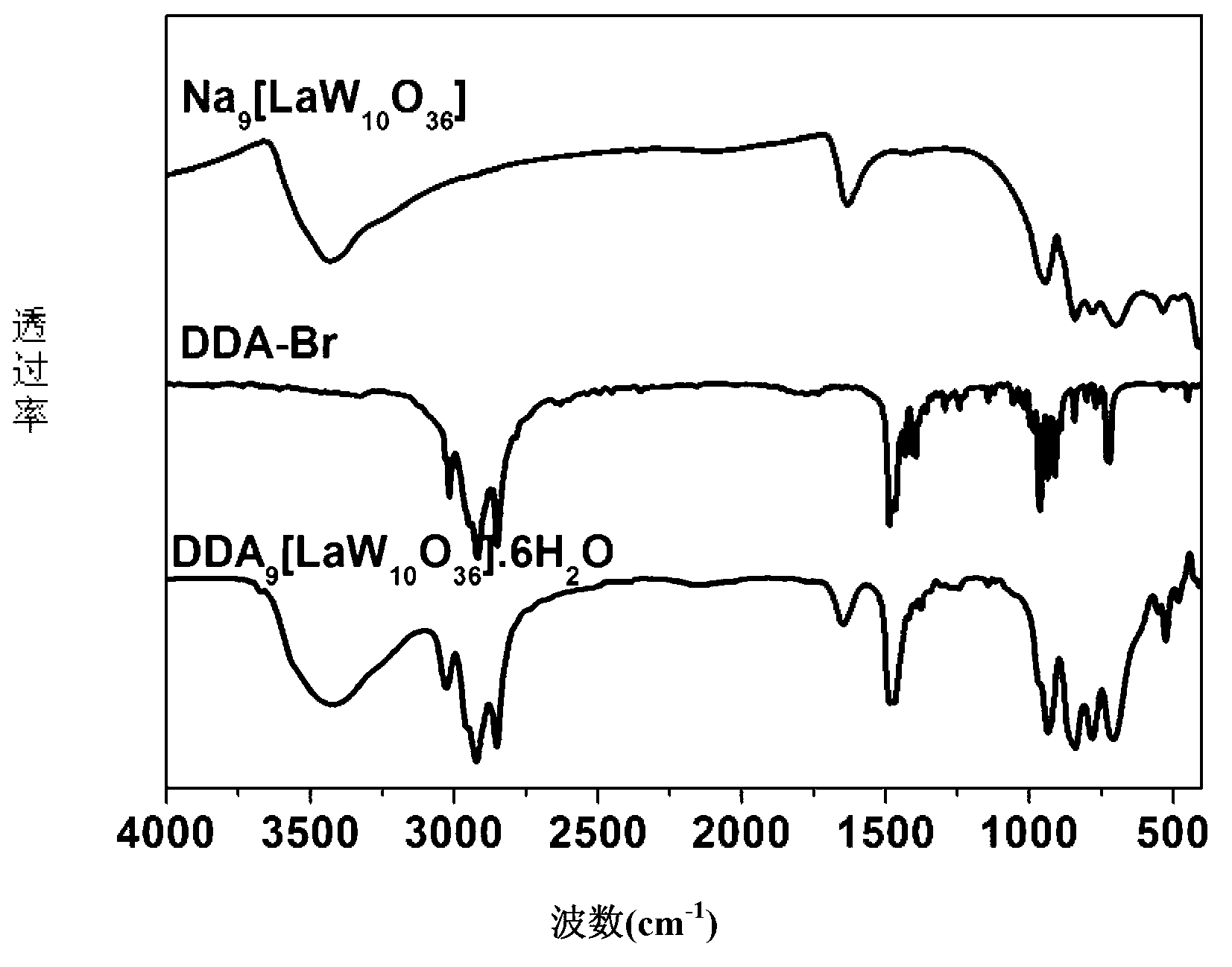
[0022] b. Stir at a temperature of 90°C to a colorless clear solution. Then the solution was cooled to room temperature, and then cooled and crystallized at 3°C to obtain Na 9 [LaW 10 o 36 ].
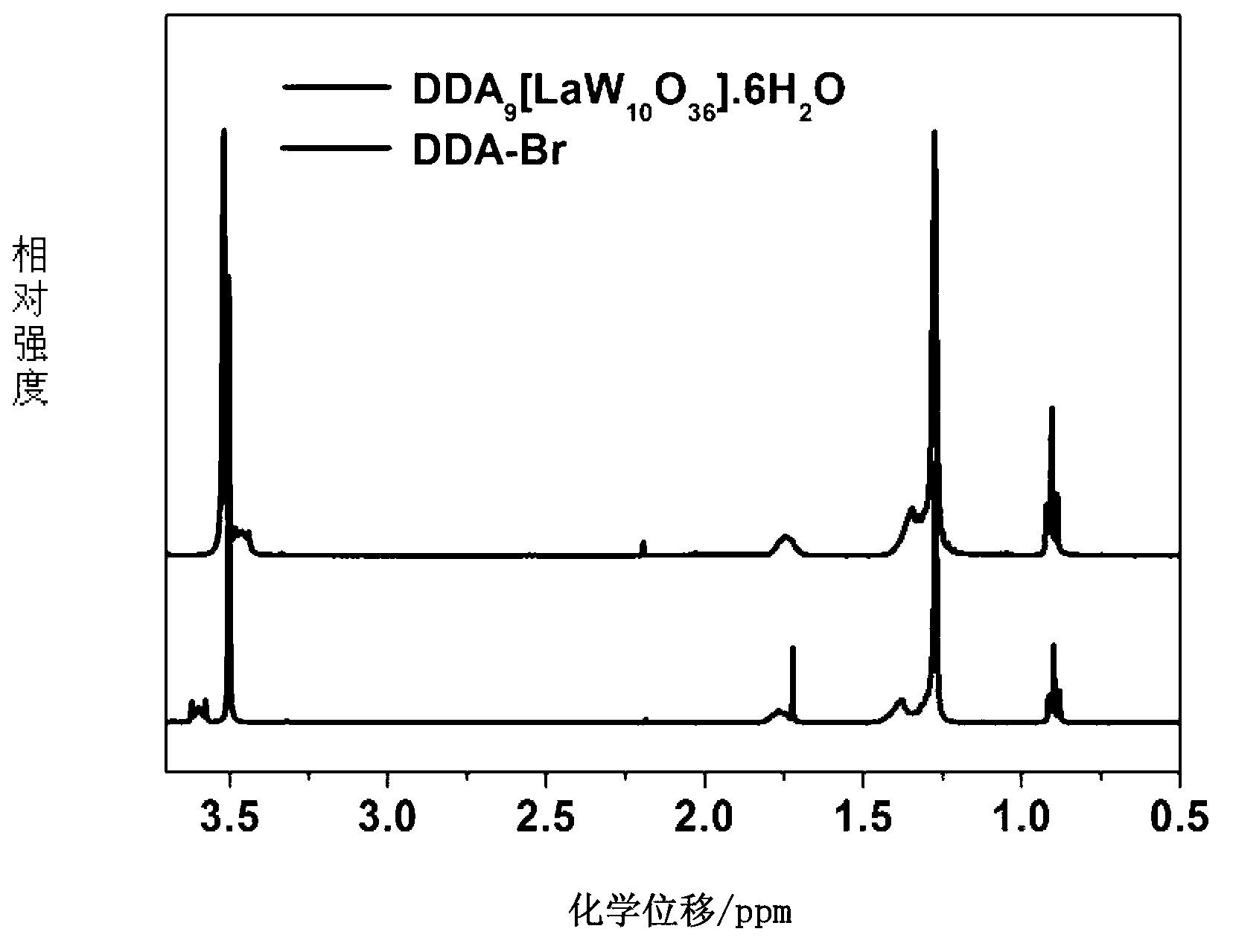
[0023] c. Take 0.27mmol of Na 9 [LaW 10 o 36 ] was dissolved in 10-20ml water, 2.3mmol of DDA-Br was dissolved in chloroform, and Na 9 [LaW 10 o 36 ] solution was dropped into chloroform solution and stirred at room temperature for 2h. ;
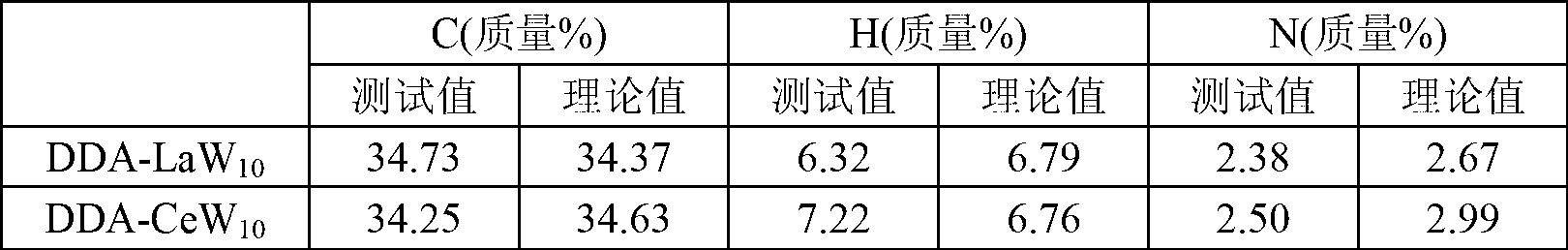
[0024] d. After the reaction is completed, add water to extract 3-5 times, take the organic layer and suspend the solution to obtain a colorless solid, which is DDA 9 [LaW 10 o 36 ].6H 2 O(DDA-LaW 10 ).
Embodiment 2
[0026] a. Add 26mmol Na 2 WO 4 2H 2O was dissolved in 20ml of water, and 2.6mmol CeCl dissolved in it was added dropwise 3 ·6H 2 In the hot aqueous solution of O, adjust the pH=7.0-7.5 with acetic acid.
[0027] b. Stir at a temperature of 90°C until a colorless clear solution is obtained. Then the solution was cooled to room temperature, and then cooled and crystallized at 3°C to obtain Na 9 [ 10 o 36 ].
[0028] c. Take 0.28mmol of Na 9 [ 10 o 36 ] was dissolved in 10-20ml water, 2.7mmol of DDA-Br was dissolved in chloroform, and Na 9 [ 10 o 36 ] solution was dropped into chloroform solution and stirred at room temperature for 2h. ;
[0029] d. After the reaction is completed, add water to extract 3-5 times, take the organic layer and suspend the solution to obtain a colorless solid, which is DDA 9 [ 10 o 36 ].4H 2 O(DDA-CeW 10 ).
[0030] Catalyzed reaction embodiment 1
[0031] The catalytic reaction is carried out in a simple glass reactor with mag...
Embodiment 2
[0039] The amphiphilic rare earth multi-acid composite material prepared in Example 1 is selected as a catalyst to carry out the catalytic reaction of epoxidation reaction to different reaction substrates. The experimental conditions are the same as those in Catalytic Reaction Example 1, and the catalytic results are shown in Table 3. As can be seen from the table, for different reaction substrates, the selectivity of the catalytic reaction is still maintained, and the conversion rate of the corresponding reaction product epoxide is greatly improved.
[0040] table 3:
[0041] catalyst
[0042] Na 9 [LaW 10 o 36 ]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com