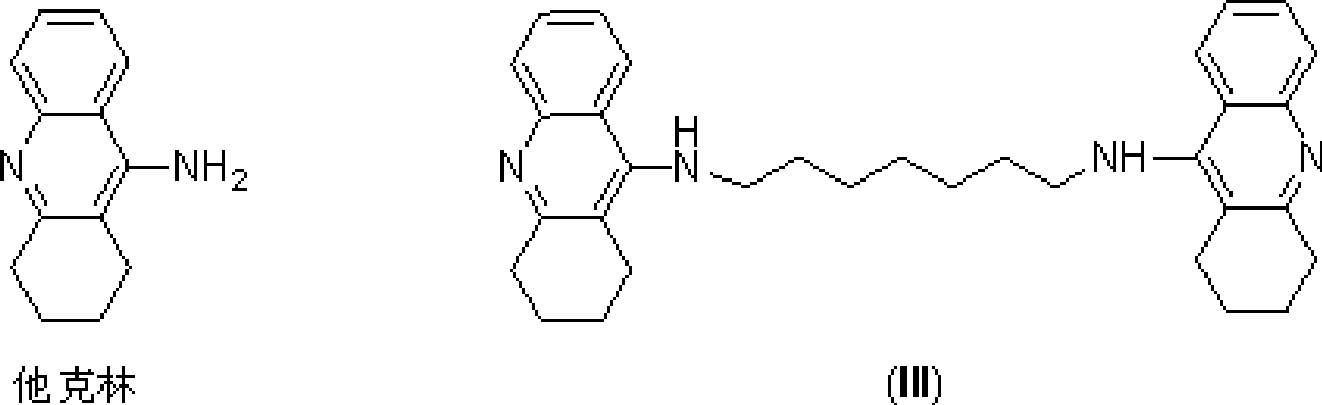
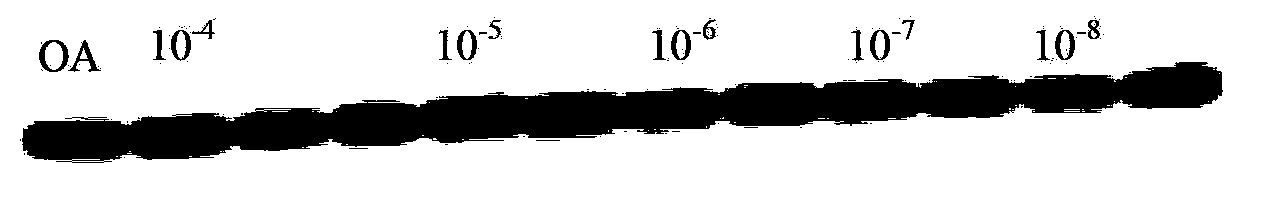Tacrine-phenothiazine isodiad compound and preparation method thereof
A phenothiazine and doublet technology, which is applied in the field of tacrine-phenothiazine heterodimer compounds, can solve the problems of unreported double inhibitory effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1: Synthesis of Intermediate A—N-(1,2,3,4-tetrahydroacridine-9-)-pentane-1,5-diamine
[0094]
[0095] Weigh 6.5g of 9-chloro-1,2,3,4-tetrahydroacridine, 12.3g of 1.5-pentanediamine, 0.45g of sodium iodide and 11.3g of phenol, mix and react at 90°C for 4 hours, and the reaction ends After cooling to room temperature, add 10wt% NaOH solution to adjust the pH value to 9-10, extract twice with ethyl acetate, combine the two extracts, wash with water, wash with saturated NaCl, anhydrous Na 2 SO 4 Dry, separate and purify by silica gel column chromatography (eluent by volume: dichloromethane / methanol / ammonia = 9:1:0.1) and concentrate to obtain a brownish-yellow oily product which is the intermediate A—N—(1,2 , 3,4-tetrahydroacridine-9-)-pentane-1,5-diamine 5.9g, yield 70%.
[0096] 1 H NMR (CDCl 3 ,300MHz) 7.90-7.97(m,2H),7.52-7.58(m,1H),7.32-7.37(m,1H),4.01(br,1H),3.45-3.52(m,2H),3.07(m, 2H),2.66-2.72(m,4H),2.01(br,2H),1.91-1.95(m,4H),1.62-1.72(m,2H),1.32-1...
Embodiment 2
[0097] Example 2: Synthesis of Intermediate A - N-(1,2,3,4-tetrahydroacridine-6-chloro-9-)-butane-1,4-diamine
[0098]
[0099] Weigh 2.5g of 6-chloro-9-chloro-1,2,3,4-tetrahydroacridine, 3.5g of 1.4-butanediamine, 0.15g of sodium iodide and 3.8g of phenol, mix and heat at 90°C for reaction After 4 hours, cool to room temperature after the reaction, add 10wt% NaOH solution to adjust the pH value to 9-10, extract twice with ethyl acetate, combine the two extracts, wash with water, wash with saturated NaCl, anhydrous Na 2 SO 4 Drying, separation and purification by silica gel column chromatography (eluent by volume ratio: dichloromethane / methanol / ammonia water = 10:1:0.1) to obtain a brownish-yellow oily product which is the intermediate A—N—(1,2,3, 2.3 g of 4-tetrahydroacridine-6-chloro-9-)-butane-1,4-diamine, yield 75%.
[0100] 1 H NMR (CDCl 3 ,300MHz) 8.03-8.10(m,1H),7.82-7.86(m,1H),7.32-7.35(m,1H),4.08(br,1H),3.15-3.23(m,2H),2.97-3.02( m,2H),2.58-2.67(m,4H),2.01(br,2H...
Embodiment 3
[0101] Example 3: Synthesis of Intermediate B—10-(2-chloroacetyl)-10H-phenothiazine
[0102]
[0103] Take 10.1g of phenothiazine, 14.7g of chloroacetyl chloride and 5.2g of triethylamine and add them into 250ml of dichloromethane, stir and reflux for 2 hours, after TLC analysis, the phenothiazine is consumed, and the reaction solution is cooled to room temperature, followed by 10wt %Na 2 CO 3 aqueous solution, 10wt% HCl aqueous solution and water washing, and then anhydrous MgSO 4 Dry, filter, and concentrate under reduced pressure to obtain the crude product, which is pre-separated and purified with 60 mesh silica gel, and the eluent containing the product is collected and concentrated, and recrystallized and purified with petroleum ether / dichloromethane = 1:1 (V / V) to obtain a white The solid 7.6g is the intermediate B—10-(2-chloroacetyl)-10H-phenothiazine, and the yield is 55%.
[0104] 1 H NMR (CDCl 3 ,300MHz)7.60(d,2H),7.48(d,2H),7.33-7.38(m,2H),7.24-7.30(m,2H),4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com