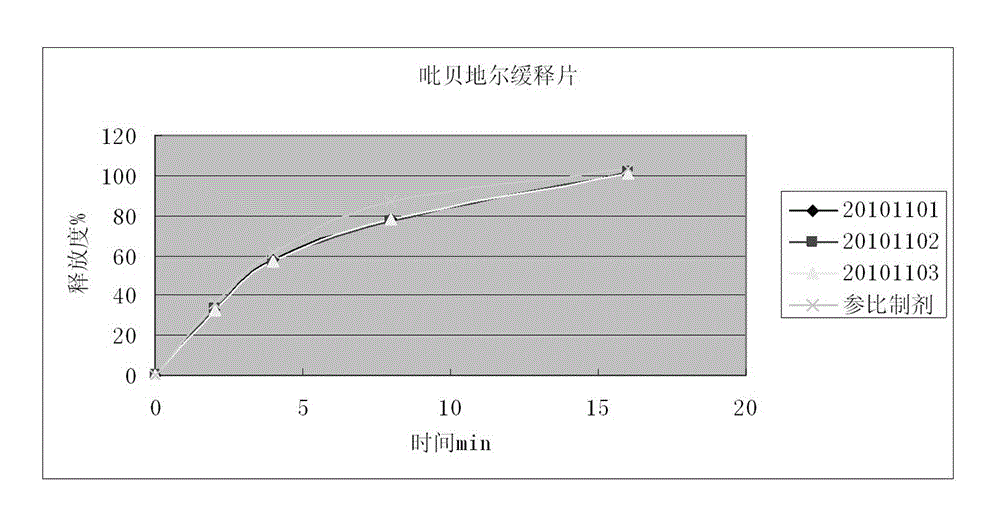
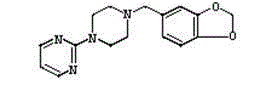
Piribedil sustained release preparation and preparation method thereof
A technology of piribedil and sustained-release preparations, which is applied in the field of biopharmaceuticals, can solve the problems of large waste of raw materials, achieve rapid absorption, improve painful symptoms, and have significant curative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The piribedil sustained-release preparation of the present invention is expressed in parts by weight g, and 1000 piribedil sustained-release preparations are prepared from 50 g of piribedil, 95 g of lactose, 34 g of hypromellose, and 60% concentration by mass. It is composed of 0.15 g of water and ethanol mixed solution and 0.5 g of magnesium stearate.
Embodiment 2
[0030] The piribedil sustained-release preparation of the present invention is expressed in parts by weight g, and 1000 piribedil sustained-release preparations are prepared from 45 g of piribedil, 105 g of lactose, 32 g of hypromellose, and 65% of mass percent concentration. It is composed of 0.12g of water and ethanol mixed solution and 0.35g of magnesium stearate.
Embodiment 3
[0032] The piribedil sustained-release preparation of the present invention is expressed in parts by weight g, and 1000 piribedil sustained-release preparations are prepared from 40 g of piribedil, 90 g of lactose, 25 g of hypromellose, and 70% of mass percent concentration. It is composed of 0.1g of water and ethanol mixed solution and 0.25g of magnesium stearate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com