Preparation technique of alogliptin benzoate
A preparation process, the technology of benzoic acid, which is applied in the field of medicine, can solve the problems of unobtainable, difficult to obtain, low purity, etc., and achieve the effects of reduced process, reduced dosage and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
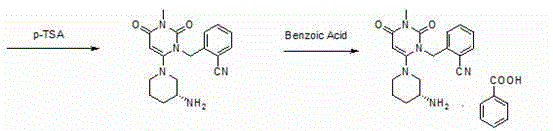
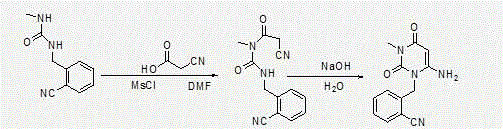
[0053] Step 1: Preparation of 2-(6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile
[0054]
[0055] (II) (III)
[0056] Add 6-chloro-3-methyluracil (80.3g, 0.5mol) and 2-cyanobenzyl bromide (107.8g, 0.55mol) into the reaction flask, add toluene 400ml, tri-n-butylamine (139g, 0.75 mol), stirred and heated to 80°C, and reacted for 5 hours. Heating was stopped, the temperature of the system was lowered to room temperature, 250 ml of purified water was added to the system, stirred for 30 minutes, filtered under reduced pressure, the filter cake was rinsed with 150 ml of purified water and then sucked dry to obtain 121 g of off-white color. Yield: 87.78%, content: 97.2%.
[0057] The 6-chloro-3-methyluracil was purchased from: Nanjing Kangmanlin Industrial Co., Ltd.
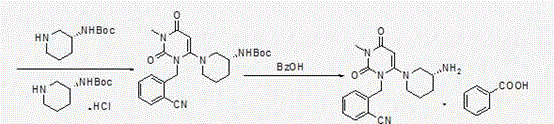
[0058] Second step: Preparation of (R)-2-[(6-(3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1( 2H)-yl)methyl]benzonitrile
[0059]
[0060] (III) (VII) (IV)
...
Embodiment 2
[0067] Basically the same as embodiment 1, on this basis:
[0068] Second step: Preparation of (R)-2-[(6-(3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1( 2H)-yl)methyl]benzonitrile
[0069] 2-(6-Chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile (110.3 g, 0.4 mol) and ( R)-3-Boc-aminopiperidine (88.1g, 0.44mol) was added to the reaction flask, 600ml of absolute ethanol and potassium carbonate (110.4g, 0.8mol) were added, stirred and heated to reflux, and refluxed for 10 hours. Potassium carbonate was removed by filtration under reduced pressure, and the filtrate was transferred to a reaction flask, p-toluenesulfonic acid (172.2 g, 1.0 mol) was slowly added, stirred and heated to reflux, and refluxed for 3 hours, a large amount of solids precipitated out of the reaction system. Filter under reduced pressure, and rinse the filter cake with 100 ml of absolute ethanol. Drained to obtain 172 g of white solid (wet product). Add all the...
Embodiment 3
[0071] Basically the same as embodiment 1, on this basis:
[0072] Second step: Preparation of (R)-2-[(6-(3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidine-1( 2H)-yl)methyl]benzonitrile
[0073] 2-(6-Chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl)-benzonitrile (110 g, 0.4 mol) and (R )-3-Boc-aminopiperidine (88g, 0.44mol) was added to the reaction flask, 600ml of absolute ethanol and potassium carbonate (55.2g, 0.4mol) were added, stirred and heated to reflux, and refluxed for 10 hours. Potassium carbonate was removed by filtration under reduced pressure, and the filtrate was transferred to a reaction flask, p-toluenesulfonic acid (103.3 g, 0.6 mol) was slowly added, stirred and heated to reflux, and refluxed for 3 hours, a large amount of solids precipitated out of the reaction system. Filter under reduced pressure, and rinse the filter cake with 100 ml of absolute ethanol. Drained to obtain 162 g of white solid (wet product). Add all the obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com