Nanogold colorimetric method for detecting mercury ions
A nano-gold colorimetric method and mercury ion technology, which is applied in the field of analytical chemistry, can solve the problems that are not conducive to popularization and application, low salinity resistance, and unfavorable environmental sample detection, etc., and achieve strong scalability, low dosage, less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of gold nanoparticles
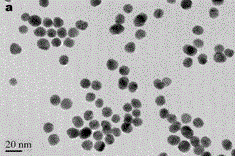
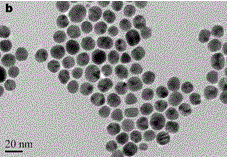
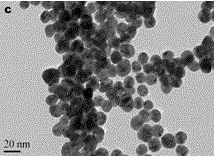
[0034] Nanogold was prepared by reducing chloroauric acid with sodium citrate (see the transmission electron micrograph for the figure 1 ). First, all glassware needs to be soaked in aqua regia to remove residual reducing substances in the glass container. Accurately weigh HAuCl 4 .4H 2 O 0.0123 g in a 250 mL three-necked flask, and then add 100 mL of water to the three-necked flask. Stir vigorously, heat to boil and reflux. Accurately weigh 0.2849 g of sodium citrate dihydrate into a 25 mL volumetric flask. Take a certain amount of sodium citrate solution, heat it in a water bath to 70 °C and quickly add it to the flask. After 15 minutes, the solution turned from colorless to purple-gray and finally wine red. Heating was continued for 10 minutes, and then heating was stopped. Stirring was continued for 10 minutes and then cooled to room temperature. The size of gold nanoparticles is related to the amount of sodium citrate ...
Embodiment 2
[0045] Compared with Example 1, the difference is only in that different concentrations of divalent mercury ions are added to the 13 nm nano-gold solution modified by mercaptoethylamine, and the ultraviolet-visible spectrum measured after 20 min; the concentration of mercury ions is as follows: ( a) 0 μmol / L; (b) 1 μmol / L; (c) 2 μmol / L; (d) 5 μmol / L; (e) 7 μmol / L; (f) 8 μmol / L; 9 μmol / L; (h) 10 μmol / L; (i) 25 μmol / L (j) 50 μmol / L; (k) 100 μmol / L;
[0046] Figure 5 based on Figure 4 Draw the standard curve for mercury ions.
Embodiment 3
[0048] Compared with Embodiment 1, the only difference is that in this embodiment:
[0049] (1) Using sodium citrate to reduce chloroauric acid to prepare wine-red nano-gold solution, the concentration of nano-gold is 1.8 nmol / L, and the particle size of nano-gold is 11 nm;
[0050] (2) Add 9 mmol / L mercaptoethylamine solution to the nano-gold solution obtained in step (1) to modify the nano-gold; wherein, the molar ratio of mercaptoethylamine to nano-gold in the mercaptoethylamine solution is 40:1 , the modification time is 15 hours;
[0051] (3) Add buffer solution to the modified nano gold solution to adjust the pH, and adjust the pH value to 7 to facilitate the formation of N-Hg 2+ -N structure; the buffer is 0.18 mol / L phosphate buffer solution.
[0052] (4) Add the mercury ion solution to be tested to the nano-gold solution obtained in step (3), mix well, and detect the UV-visible spectrum of the solution after 15 minutes to obtain the UV-visible spectrum correspondin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com