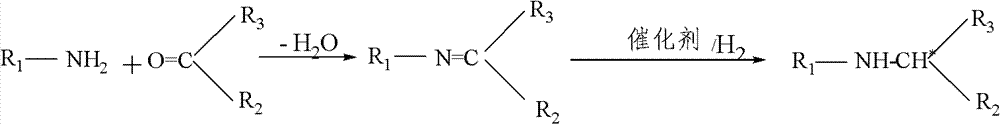
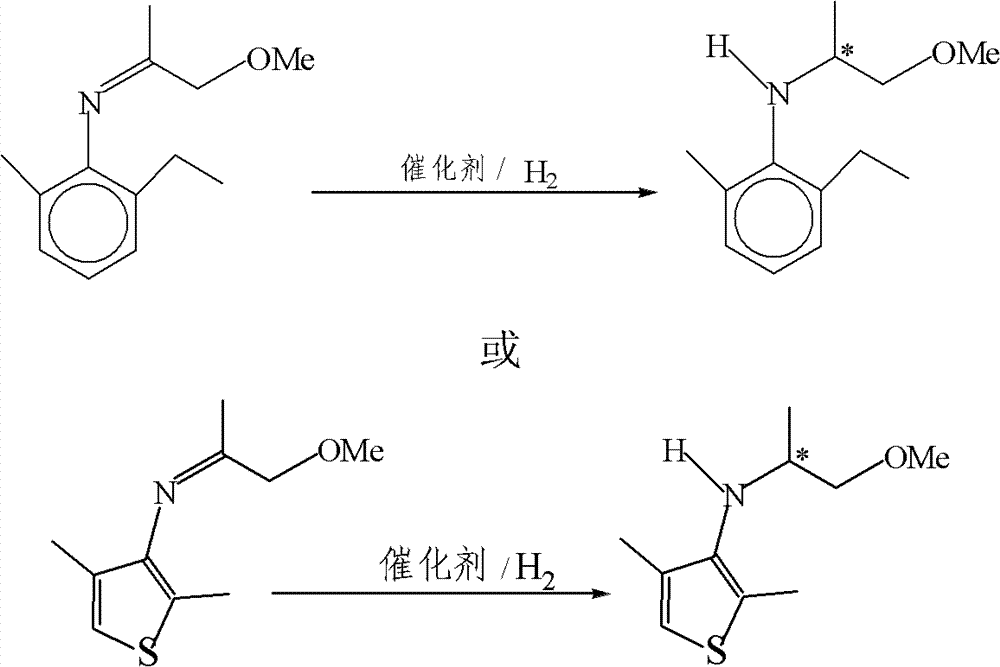
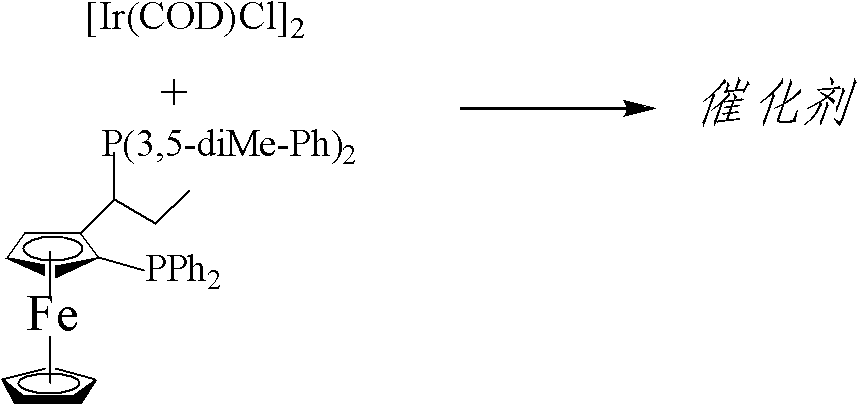Asymmetric catalytic hydrogenation method of imine
A catalytic hydrogenation, asymmetric technology, applied in asymmetric synthesis, organic chemistry methods, chemical instruments and methods, etc., can solve the problems such as difficulty in obtaining chiral alcohol intermediates, difficulty in meeting synthetic needs, time-consuming, etc. The effect of industrial practical value, easy preparation and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Measure 5mL (containing ligand 12mg, [Ir(COD)Cl] 2 5mg, Bu 4 NI 125mg, s / c=29,500), add it into a 100mL autoclave under the protection of nitrogen, and add 44.5g of imine under the protection of nitrogen, and then replace it with hydrogen at 10 atmospheres for 3 times; pass through hydrogen at 80 atmospheres, and heat up to 50 DEG C, stopped reaction after 5 hours, analyzed by gas chromatography, the conversion rate of raw materials was 100%, distilled under reduced pressure to obtain product 40.5g, yield 90.1%, analyzed by high performance liquid chromatography, e.e value was 76.5%.
Embodiment 2
[0031] Other conditions are the same as in Example 1, change the reaction temperature to be room temperature, stop the reaction after 24 hours of reaction, analyze with gas chromatography, the conversion rate of raw materials is 98.7%, and distill under reduced pressure to obtain 41.3g of product, and the yield is 91.9% and analyze with high performance liquid chromatography , e.e value is 78.5%.
Embodiment 3
[0033] Other conditions are identical with embodiment 2, logical 60 atmospheric pressure hydrogen, stop reaction after reacting for 24 hours, with gas chromatographic analysis raw material is all converted, with high performance liquid chromatographic analysis, e.e value is 77.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com