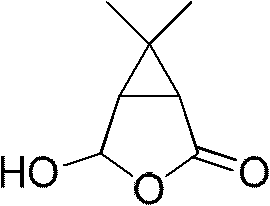
New synthetic method of carane aldehyde acid lactone, caronic acid, caronic anhydride and key intermediates thereof
A technology of carolic acid lactone and synthesis method, which is applied in chemical instruments and methods, preparation of carboxylic acid by oxidation, preparation of oxygen-containing compounds, etc., can solve problems such as large amount of acetone usage, dangerous operation, increased production cost, etc., and achieve easy Industrialized production, high production safety and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0056] Preparation of Intermediate IIIa:
[0057]
[0058] Add intermediate la (54g) in the reaction flask, copper catalyst (the catalyst is the complex of cuprous trifluoromethanesulfonate and benzonitrile 1:1) (0.5g), dichloroethane 200ml, heat up to 60-65 ℃. A solution of 48 g of ethyl diazoacetate II dissolved in 100 ml of dichloroethane was added dropwise to the system, and the drop was completed while maintaining an internal temperature of 60-85°C. Stirring was continued for 30 minutes after dropping. Distilled under reduced pressure, collected fractions at 117-120°C / 1kPa to obtain 60g of intermediate IIIa.
[0059] Trans IIIa NMR:
[0060] 1 HNMR δ4.18(dd, J=12, 7Hz 1H), 4.13(m, 2H), 4.00(dd, J=12, 8Hz, 1H), 2.07(s, 3H), 1.73(ddd, J=8, 7, 5.5Hz, 1H), 1.43(d, J=5.5Hz, 1H), 1.27(s, 3H), 1.26(t, J=7Hz, 3H), 1.19(s, 3H)
[0061] Cis IIIa NMR:
[0062] 1 HNMR: δ4.50(dd, J=12, 7Hz, 1H), 4.39(dd, J=12, 8Hz, 1H), 4.11(m, 2H), 2.06(s, 3H), 1.60(d, J= 9Hz, 1H), 1.44(d...
Embodiment 1b
[0064] Preparation of Intermediate IIIb:
[0065]
[0066] Operation is the same as example Ia: wherein catalyzer is the complex compound that is cuprous trifluoromethanesulfonate and benzonitrile 1: 1, and the weight ratio of catalyzer and raw material Ib is 0.01, and the mol ratio of raw material Ib and ethyl diazoacetate II is 1:1.5.
[0067] Trans IIIb NMR:
[0068] 1 HNMR: δ4.12 (m, 2H), 3.74 (dd, J=11, 6Hz, 1H), 3.53 (dd, J=11, 8Hz, 1H), 1.66 (ddd, J=8, 6, 5.5Hz, 1H), 1.33(d, J=5Hz, 1H), 1.26(t, J=7Hz, 3H), 1.23(s, 3H), 1.18(s, 3H), 0.10(s, 9H)
[0069] Cis IIIb NMR:
[0070] 1 HNMR: δ4.08(m, 2H), 3.91(dd, J=6.5, 2.5Hz, 2H), 1.52(d, J=9Hz, 1H), 1.40(ddd, J=9, 6.52.5Hz, 1H) , 1.25(s, 3H), 1.25(t, J=7Hz, 3H), 1.18(s, 3H), 0.10(s, 9H)
Embodiment 1c
[0072] Preparation of Intermediate IIIc:
[0073]
[0074] Operation is the same as example Ia: wherein catalyzer is the complex compound of cuprous trifluoromethanesulfonate and benzonitrile 1: 1, and the weight ratio of catalyzer and raw material Ic is 0.01, and the mol ratio of raw material Ic and ethyl diazoacetate II is 1 : 1.5.
[0075] Trans IIIc NMR:
[0076] 1 HNMR: δ4.12 (m, 2H), 3.77 (dd, J=11, 6Hz, 1H), 3.55 (dd, J=11, 8Hz, 1H), 1.63 (ddd, J=9, 6, 5Hz, 1H ), 1.35(d, J=5Hz, 1H), 1.24(t, J=7Hz, 3H), 1.22(s, 3H), 1.18(s, 3H), 0.89(s, 9H), 0.06(s, 3H ), 0, 05(s, 3H)
[0077] Cis IIIc NMR:
[0078] 1 HNMR: δ4.08(m, 2H), 3.96(dd, J=11, 7Hz, 1H), 3.90(dd, J=11, 6.5Hz, 1H), 1.52(d, J=9Hz, 1H), 1.40 (ddd, J=9, 7, 6.5Hz, 1H), 1.25(s, 3H), 1.25(t, J=7Hz, 3H), 1.18(s, 3H), 0.89(s, 9H), 0.06(s , 3H), 0.05(s, 3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com