Pyrrole ionic liquid, and preparation method and application thereof
A technology of ionic liquid and pyrrole, which is applied in the field of supercapacitors, pyrrole-based ionic liquids and their preparation, can solve problems such as easy decomposition, reduced capacitance, and increased internal resistance of capacitors, achieving good thermal stability, high decomposition voltage, The effect of high conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] A preparation method of the above-mentioned pyrrole-based ionic liquid, the preparation process is as follows:
[0040] N-Methylpyrrole→N-Alkyl-N-Methylpyrrole Halide→Pyrrole Cationic Ionic Liquid
[0041]
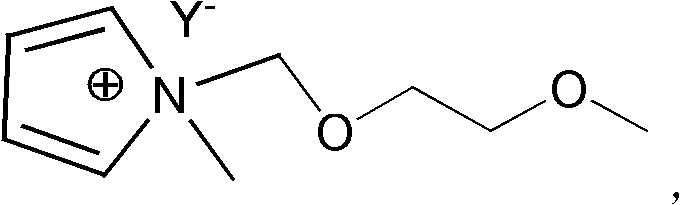
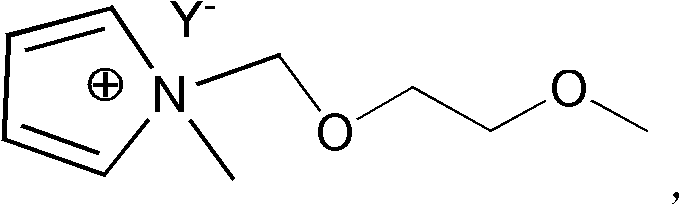
[0042] where RX represents haloalkane or In the inorganic salt MY containing an organic anion, the cation M + Can be Na + 、K+ and NH 4 + etc.; anion Y - Can be BF 4 - 、PF 6 - , (CF 3 SO 2 ) 2 N - 、CF 3 SO 3 - or (FSO 2 ) 2 N - Wait.
[0043] Specifically include the following steps:
[0044] Step S1: react N-methylpyrrole with methoxyethoxyhalomethane in a molar ratio of 1:1 to 1:1.2 to generate N-methoxyethoxymethyl-N-methylpyrrole halide Salt.
[0045] The details are as follows: mix N-methylpyrrole and methoxyethoxyhalomethane in a molar ratio of 1:1 to 1:1.2, under the protection of an inert gas, heat up to 60-80°C, stir and react, then cool to room temperature , washed with ethyl acetate and dried in vacuo to give N-methoxyethoxymet...
Embodiment 1
[0063] Example 1: Synthesis of N-methoxyethoxymethyl-N-methylpyrrole tetrafluoroborate
[0064] 81g, 1mol of N-methylpyrrole and 136.4g, 1.1mol of methoxyethoxymethane chloride were respectively added into a 250mL flask, and the temperature was raised to 60°C under a nitrogen atmosphere, and the reaction was stirred for 72 hours. Let stand to cool, and wash three times with ethyl acetate. Vacuum drying at 80° C. gave a pale yellow solid, namely N-methoxyethoxymethyl-N-methylpyrrole chloride, with a yield of 83%.
[0065] Add 102.5g, 0.5mol of N-methoxyethoxymethyl-N-methylpyrrole chloride, 55g, 0.5mol of NaBF to a 500mL flask 4 and 100 mL of deionized water, stirred at room temperature for 8 hours. After the reaction was completed, the mixture was extracted three times with 250 mL of dichloromethane, and the extracts were combined. Then back-extracted with 60 mL deionized water each time until saturated with AgNO 3 The aqueous solution was titrated until no precipitation o...
Embodiment 2
[0067] Example 2: Synthesis of N-methoxyethoxymethyl-N-methylpyrrole hexafluorophosphate
[0068] 81g, 1mol of N-methylpyrrole and 184.8g, 1.1mol of methoxyethoxymethyl bromide were respectively added into a 250mL flask. Under an argon atmosphere, the temperature was raised to 80°C, and the reaction was stirred for 48 hours. Let stand to cool, and wash three times with ethyl acetate. Vacuum drying at 80° C. gave a pale yellow solid, N-methoxyethoxymethyl-N-methylpyrrole bromide, with a yield of 86%.
[0069] Add 0.5mol N-methoxyethoxymethyl-N-methylpyrrole bromide, 92g, 0.5mol KPF to a 500mL flask 6 and 125 mL of deionized water, stirred at room temperature for 16 hours. After the reaction was completed, the mixture was extracted three times with 250 mL of dichloromethane, and the extracts were combined. Then back-extracted with 60 mL deionized water each time until saturated with AgNO 3 The aqueous solution was titrated until no precipitation occurred in the aqueous phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com