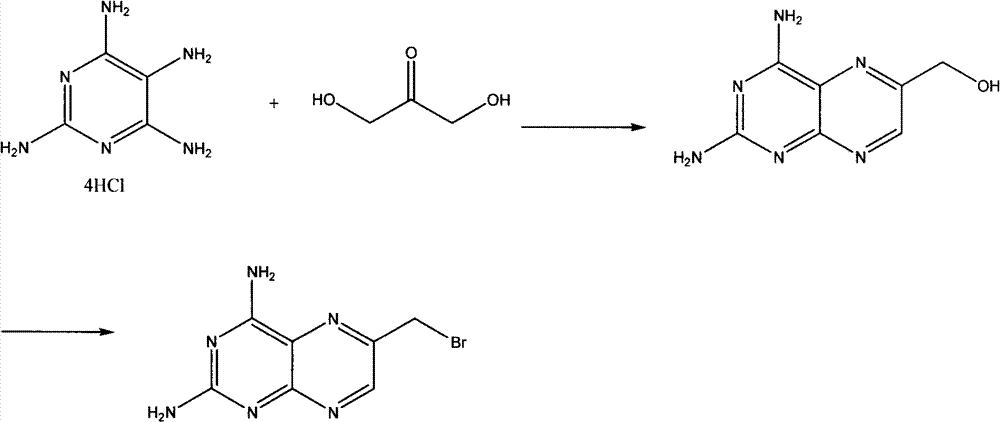
Preparation method of 2,4-diamino-6-bromomethyl pteridine
A technology of bromomethylpteridine and hydroxymethylpteridine, applied in the field of compound preparation, can solve the problems of low yield, cumbersome route, difficult to enlarge, etc., and achieves the effects of high product purity, simple post-processing, and avoiding deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 28.6g of 2,4,5,6-tetraaminopyrimidine hydrochloride, 500mL methanol and water mixed solution (v:v=1:1) into a 1L four-neck flask, stir to dissolve. Add 50 g of acidic 4A molecular sieves, pass air under vigorous stirring, and react for 12 hours. Molecular sieves were removed by filtration, the filtrate was adjusted to pH=8 with sodium bicarbonate, a brown solid was precipitated, filtered, and dried to obtain 14 g of 2,4-diamino-6-hydroxymethylpteridine, yield: 73%. MS m / z 192(M+H) +
Embodiment 2
[0018] Add 28.6g of 2,4,5,6-tetraaminopyrimidine hydrochloride, 500mL methanol and water mixed solution (v:v=1:1) into a 1L four-neck flask, stir to dissolve. Add 50 g of acidic 4A molecular sieves, feed oxygen under vigorous stirring, and react for 12 hours. Molecular sieves were removed by filtration, and the filtrate was adjusted to pH=8 with sodium bicarbonate, and a brown solid was precipitated, filtered, and dried to obtain 17.5 g of 2,4-diamino-6-hydroxymethylpteridine, yield: 91%. MS m / z 192(M+H) +
Embodiment 3
[0020] Add 26g of triphenylphosphine, 18g of NBS, and 200mL of carbon tetrachloride into a 500mL four-necked flask, stir vigorously and cool down to 0°C. Add 10 g of 2,4-diamino-6-hydroxymethylpteridine in batches, and then stir the reaction overnight. Filter to obtain the crude product. 12.8 g of 2,4-diamino-6-bromomethylpteridine was recrystallized from a mixed solvent of 100 mL of water and DMF to obtain 12.8 g of brown needle crystals, with a yield of 96%. MS m / z254(M+H) +1 H NMR (400MHz, d6-DMSO) δ: 9.10(s, 1H), 8.65(s, 2H), 8.01(s, 2H), 4.94(s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com