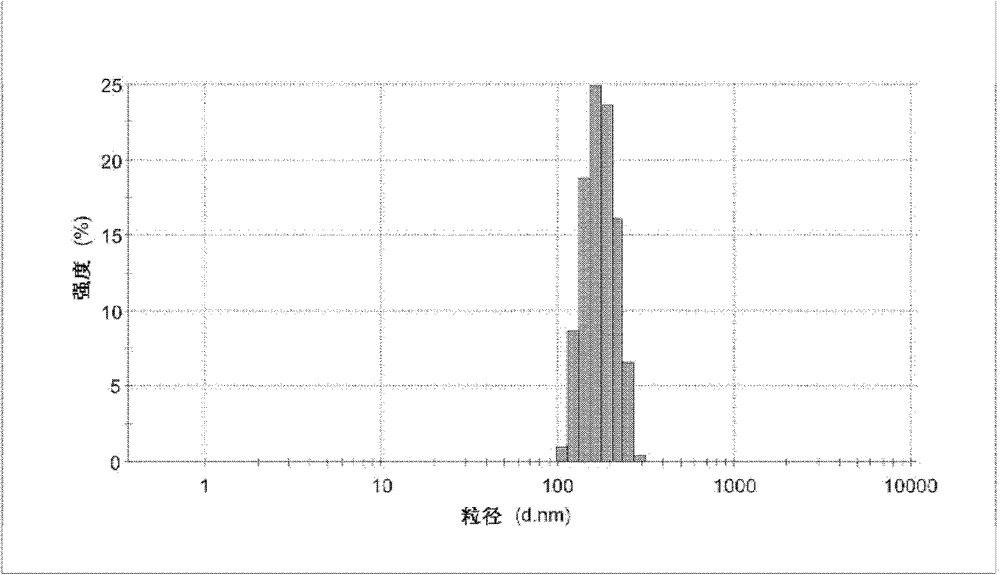
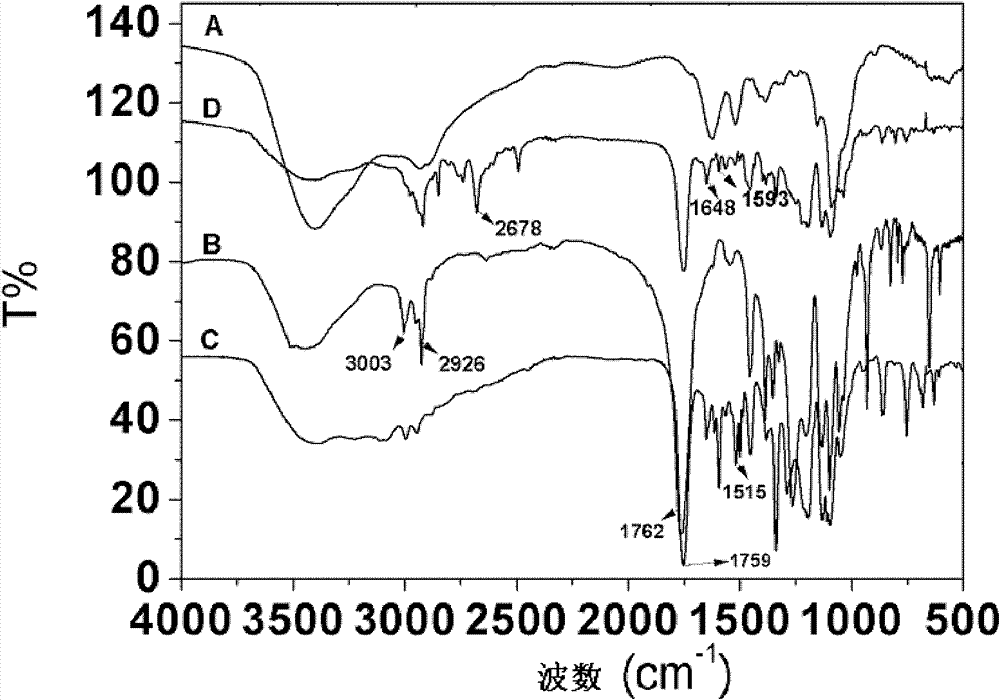
Graft polymer, and preparation method and use thereof
A technology of grafted polymers and products, which can be used in pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as poor hydrophilicity, reduced compatibility, and reduced degradation rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of chitosan-polylactic acid-dipalmitoylphosphatidylethanolamine graft copolymer
[0067] (1) Disperse 10g of chitosan (molecular weight: 200,000) in water and heat to 75°C. 7ml of 30% H was added dropwise with stirring 2o 2 , Reaction 2h. Cool and filter. Part of the water in the filtrate was rotary evaporated, then precipitated in absolute ethanol, and left overnight. Suction filtration is then obtained to obtain pale yellow soluble chitosan.
[0068] Add 1 g of dried soluble chitosan into 25 ml of dimethyl sulfoxide, and disperse for 1 h, in which nitrogen is passed through for 0.5 h, and vacuum is pumped for 0.5 h. 20 g of lactide (Alfar Aesar company, 97%, analytically pure) was added to the chitosan solution, and dispersed for 1 h under nitrogen protection. Then add 1.5ml of triethylamine, under the protection of nitrogen, react at 85°C for 10h, then precipitate with 300ml of water, wash with water 4 times, dry in a vacuum oven for 24 hours, then ex...
Embodiment 2
[0086]Synthesis of Chitosan-Polyglycolide-Dioleoylphosphatidylethanolamine Copolymer
[0087] (1) Disperse 10g of chitosan (molecular weight: 200,000) in water and heat to 75°C. 7ml of 30% H was added dropwise with stirring 2 o 2 , Reaction 2h. Cool and filter. Part of the water in the filtrate was rotary evaporated, then precipitated in absolute ethanol, and left overnight. Suction filtration is then obtained to obtain light yellow soluble chitosan.
[0088] Add 1 g of dried soluble chitosan into 4 ml of dimethyl sulfoxide, and disperse for 1 h, in which 0.5 h is passed through nitrogen, and 0.5 h is vacuumized. 1 g of glycolide (Alfar Aesar company, 97%, analytically pure) was added to the chitosan solution, and dispersed for 1 h under nitrogen protection. Add 0.1ml of triethylamine, under the protection of nitrogen, react at 70°C for 10h, then precipitate with 300ml of water, wash with water 4 times, dry in a vacuum oven for 24 hours, and then extract with toluene (30...
Embodiment 3
[0094] Synthesis of chitosan-polycaprolactone-distearoylphosphatidylethanolamine graft copolymer
[0095] (1) Disperse 10g of chitosan (molecular weight: 200,000) in water and heat to 75°C. 7ml of 30% H was added dropwise with stirring 2 o 2 , Reaction 2h. Cool and filter. Part of the water in the filtrate was rotary evaporated, then precipitated in absolute ethanol, and left overnight. Suction filtration is then obtained to obtain light yellow soluble chitosan.
[0096] Add 1 g of dried soluble chitosan into 100 ml of dimethyl sulfoxide, and disperse for 1 h, in which nitrogen is passed through for 0.5 h, and vacuum is pumped for 0.5 h. Add 30 g of caprolactone (Alfar Aesar company, 97%, analytically pure) into the chitosan solution, and disperse for 1 h under nitrogen protection. Add 5ml of triethylamine, under the protection of nitrogen, react at 85°C for 15h, then precipitate with 300ml of water, wash with water 4 times, dry in a vacuum oven for 24 hours, and then ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com