Production method of dihydromyrcenol
A technology for dihydromyrcenol and dihydromyrcene, which is applied in the field of producing dihydromyrcenol, can solve the problems of low conversion rate, high energy consumption, poor selectivity, etc. The effect of energy saving and isomerization suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
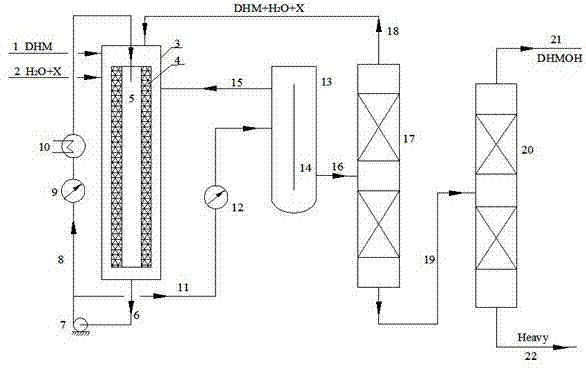
[0035]In the catalyst fixed bed layer 4 of the IRRFB reactor 3, the catalyst strong acid ion exchange resin Amberlyst15 (provided by Rohm and Haas company in the United States) is filled, and dihydromyrcene, water and chloroform are respectively input through the DHM feed pipe 1 and water and solvent The tube 2 enters the forced circulation radiant flow fixed bed (IRRFB for short) reactor 3, the mass ratio of DHM, water and chloroform solvent is 1:1:1, and it is preheated by the preheater to reach the reaction temperature of 60°C respectively. The pressure is 0.2MPa, and the catalyst loading in the catalyst fixed bed 4 is 25% of the reactor volume. The distance between the outer wall surface of the catalyst fixed bed 4 and the inner wall surface of the reactor is 60mm. The central pipe 5 has the same diameter as the second pipe 8 . The catalyst thickness of the catalyst fixed bed 4 (approximately equal to the distance between the inner wall and the outer wall) is 100 mm. The...
Embodiment 2
[0040] In the catalyst fixed bed layer 4 of the IRRFB reactor 3, the catalyst strong acid ion exchange resin Amberlyst36 (provided by Rohm and Haas, USA) is filled, and dihydromyrcene, water and chloroform are input through the DHM feed pipe 1 and water and solvent respectively Tube 2 enters the forced circulation radiant flow fixed bed (hereinafter referred to as IRRFB) reactor 3, the mass ratio of DHM, water and chloroform solvent is 1:0.5:1, and it is preheated by the preheater to reach the reaction temperature of 58°C respectively. The pressure is 0.18MPa, and the catalyst loading in the catalyst fixed bed 4 is 25% of the reactor volume. The distance between the outer wall surface of the catalyst fixed bed 4 and the inner wall surface of the reactor is 60mm. The central pipe 5 has the same diameter as the second pipe 8 . The catalyst thickness of the catalyst fixed bed 4 (approximately equal to the distance between the inner wall and the outer wall) is 100 mm. The overal...
Embodiment 3
[0044] In the catalyst fixed bed 4 of the IRRFB reactor 3, the catalyst strong acid ion exchange resin Amberlyst15 is packed, and dihydromyrcene, water and methyl ethyl ketone enter into the forced In the circulating radiant flow fixed bed (IRRFB for short) reactor 3, the mass ratio of DHM, water and methyl ethyl ketone solvent is 1:0.5:1, and they are preheated by the preheater respectively to reach the reaction temperature of 73°C. The pressure is 0.25MPa, and the catalyst loading in the catalyst fixed bed 4 is 25% of the reactor volume. The distance between the outer wall surface of the catalyst fixed bed 4 and the inner wall surface of the reactor is 60mm. The central pipe 5 has the same diameter as the second pipe 8 . The catalyst thickness of the catalyst fixed bed 4 (approximately equal to the distance between the inner wall and the outer wall) is 100mm. The overall height of the reactor 3 is typically 1600 mm. The flow rate of the circulation pump is V= 50V 0 .
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com