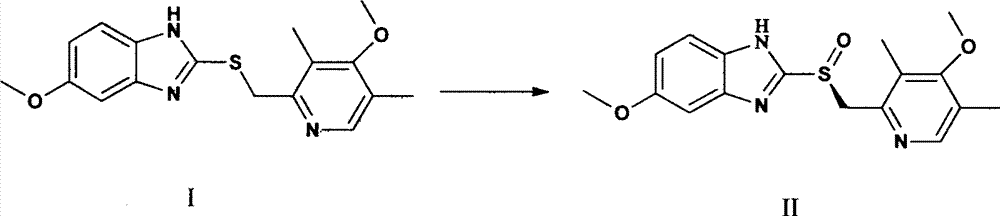
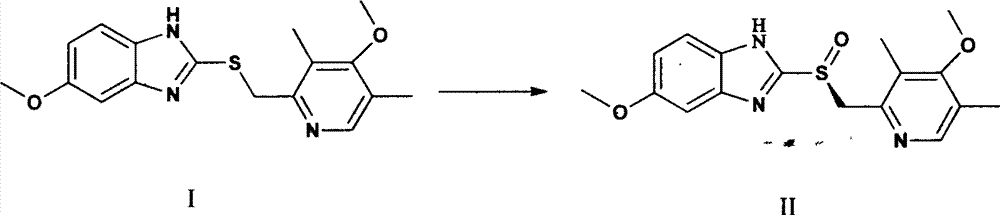
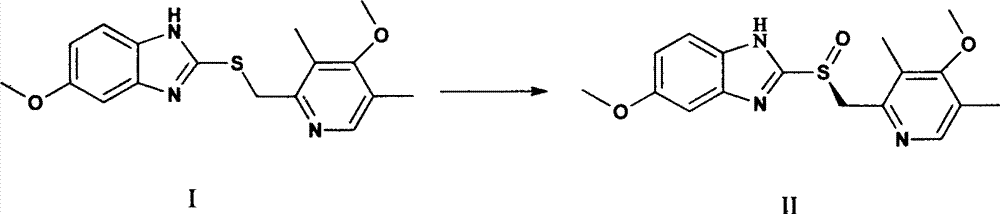
Production method of high-optical-purity esomeprazole
A technology of esomeprazole and benzimidazole, applied in the production field of high optical purity esomeprazole salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019] Weigh 9 kg of sulfide shown in formula I, add 35 L of tetrahydrofuran, add 3 L of D-diethyl tartrate, 2.5 L of tetraisopropyl titanate under stirring, slowly raise the temperature to 35 ° C, stir for 90 minutes, and dropwise add triethylamine 1.4 L, lower the temperature to -10°C, add dropwise a solution of 5.2kg of sodium m-chloroperoxybenzoate dissolved in 8L of water, continue stirring for 60 minutes after the dropwise addition, add 25L of 10% ammonia water to the reaction solution, stir, and wash the water with 10L of toluene Phase once, separate the water phase, slowly add acetic acid dropwise to adjust pH = 7.5 ~ 8.0, extract twice with 20 L of dichloromethane, combine the dichloromethane, and remove the solvent under reduced pressure at 40 ° C to obtain a brown oily substance, which is dissolved in 10 L Add dropwise 20 L of 0.5% sodium bicarbonate aqueous solution to acetone, stir to obtain a white solid, and air-dry at 40° C. to obtain 5.1 kg of esome...
Embodiment 2
[0022] Weigh 9kg of sulfide shown in formula I, add 35L of tetrahydrofuran, add 3L of D-diethyl tartrate and 2.5L of tetraisopropyl titanate under stirring, slowly raise the temperature to reflux at 65°C, stir for 30min, add diisopropyl 1.8 L of ethyl ethylamine, cooled to 0 ° C, dropwise added a solution of 5.2 kg of sodium m-chloroperoxybenzoate dissolved in 8 L of water, continued to stir for 60 min after the dropwise addition, added 15 L of 25% ammonia water to the reaction solution, stirred, and Wash the water phase once with 10L of toluene, separate the water phase, slowly add acetic acid dropwise to adjust the pH=8.0-8.5, extract twice with 20L of ethyl acetate, combine the organic phases, and remove the solvent under reduced pressure at 40°C to obtain a brown oil. Dissolve in 10L of acetone, add 20L of 0.25% sodium bicarbonate solution dropwise, stir to obtain a white solid, and air-dry at 40°C to obtain 4.9kg of esomeprazole, which can be used to prepare esomeprazole s...
Embodiment 3
[0025] Weigh 18 kg of the sulfide shown in formula I, add 65 L of tetrahydrofuran, add 6.2 L of D-diethyl tartrate and 5.0 L of tetraisopropyl titanate under stirring, slowly raise the temperature to 65°C and reflux, stir for 60 minutes, and dropwise add diisopropyl titanate Propylethylamine 3.1L, cooled to 5°C, dropwise added a solution of 10.0kg sodium m-chloroperoxybenzoate dissolved in 13L water, continued to stir for 60min after the dropwise addition, added 45L of 12.5% ammonia water to the reaction solution, stirred, Wash the water phase once with 20L of toluene, separate the water phase, slowly add acetic acid dropwise to adjust the pH=8.0~8.5, extract twice with 40L of dichloromethane, combine the organic phase, and remove the solvent under reduced pressure at 40°C to obtain a brown oily substance , dissolved in 20L of acetone, added dropwise to 35L of 0.2% sodium bicarbonate aqueous solution, stirred to obtain a white solid, and air-dried at 40°C to obtain 10.7kg of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com