Florfenicol solid dispersoid and preparation method thereof
A technology of solid dispersion and florfenicol, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problems of inability to industrialize promotion, high cost, complicated operation process, etc. , to achieve the effect of rapid and reliable drug effect, low drug residue and simple operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
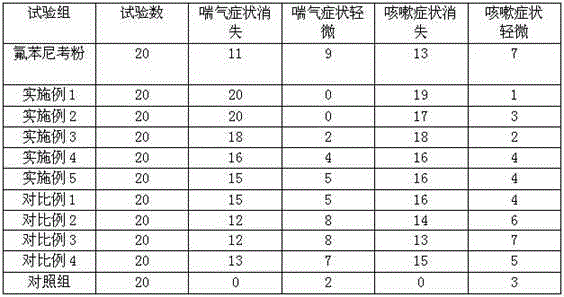
Examples
Embodiment 1
[0018] A florfenicol solid dispersion, comprising the following components by weight ratio: 12 parts of florfenicol, 48 parts of carrier;
[0019] Mix the above components well, put them in a high-pressure reactor, turn on the constant temperature circulating water bath and the metering pump, put CO2 into the heater through the buffer tank and raise the temperature to 42°C, open the intake valve, turn on the compressor, and CO2 enters the reactor , maintain a constant pressure of 17.5Mpa in the kettle, so that the excipient carrier and the drug can fully react under the action of pressure, and combine them by hydrogen bonds. Dispersion particles, and then carry out ultrafine grinding of the solid dispersion, and pass through a 400-mesh sieve to obtain a finished product;
[0020] Wherein, the carrier is composed of polyvinylpyrrolidone, polyethylene glycol 6000, mannitol, carboxypropyl methylcellulose, and β-cyclodextrin, and the weight ratio is polyvinylpyrrolidone: polyethyl...
Embodiment 2
[0022] A florfenicol solid dispersion, comprising the following components by weight ratio: 10 parts of florfenicol, 40 parts of carrier;
[0023] Mix the above components well, put them in a high-pressure reactor, turn on the constant temperature circulating water bath and the metering pump, put CO2 into the heater through the buffer tank and raise the temperature to 35°C, open the intake valve, turn on the compressor, and CO2 enters the reactor , maintain a constant pressure of 11.5Mpa in the kettle, so that the excipient carrier and the drug can fully react under the action of pressure, and combine them by hydrogen bonding. The reaction time is 5 hours. After rapid decompression, the reaction kettle is opened, and the solid dispersion prepared in the kettle is collected solid particles, and then ultrafinely pulverize the solid dispersion, and pass through a 400-mesh sieve to obtain a finished product;
[0024] Wherein, the carrier is composed of polyvinylpyrrolidone, polyet...
Embodiment 3
[0026] A florfenicol solid dispersion, comprising the following components by weight ratio: 15 parts of florfenicol, 60 parts of carrier;
[0027] Mix the above components well, put them in a high-pressure reactor, turn on the constant temperature circulating water bath and the metering pump, put CO2 into the heater through the buffer tank and raise the temperature to 60°C, open the intake valve, turn on the compressor, and CO2 enters the reactor , maintain a constant pressure of 25.5Mpa in the kettle, so that the excipient carrier and the drug can fully react under the action of pressure, and combine them by hydrogen bonding. The reaction time is 8 hours. After rapid decompression, the reaction kettle is opened, and the solid dispersion prepared in the kettle is collected. solid particles, and then ultrafinely pulverize the solid dispersion, and pass through a 400-mesh sieve to obtain a finished product;
[0028] Wherein, the carrier is composed of polyvinylpyrrolidone, polye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com