Low carbon alkane dehydrogenation olefin production catalyst and preparation method thereof
A low-carbon alkane and catalyst technology, which is applied in the field of catalysts for dehydrogenation of low-carbon alkanes to olefins and their preparation, can solve the problems of frequent regeneration, energy consumption, rapid deactivation, etc. The method is simple and easy to implement, and the effect of prolonging the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
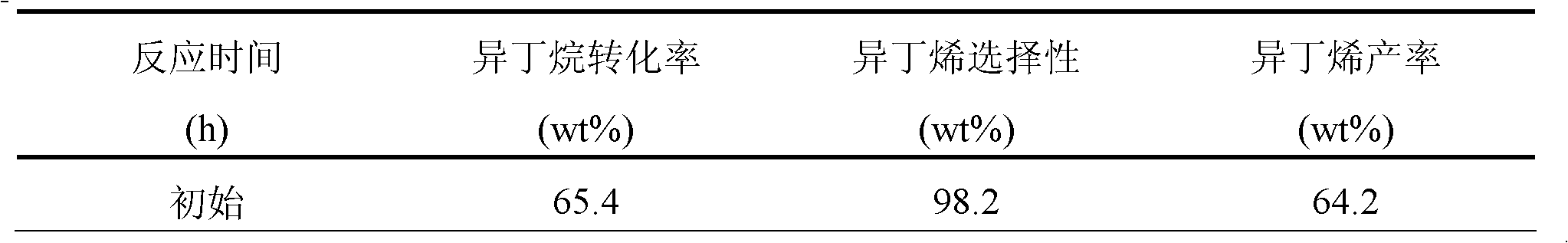
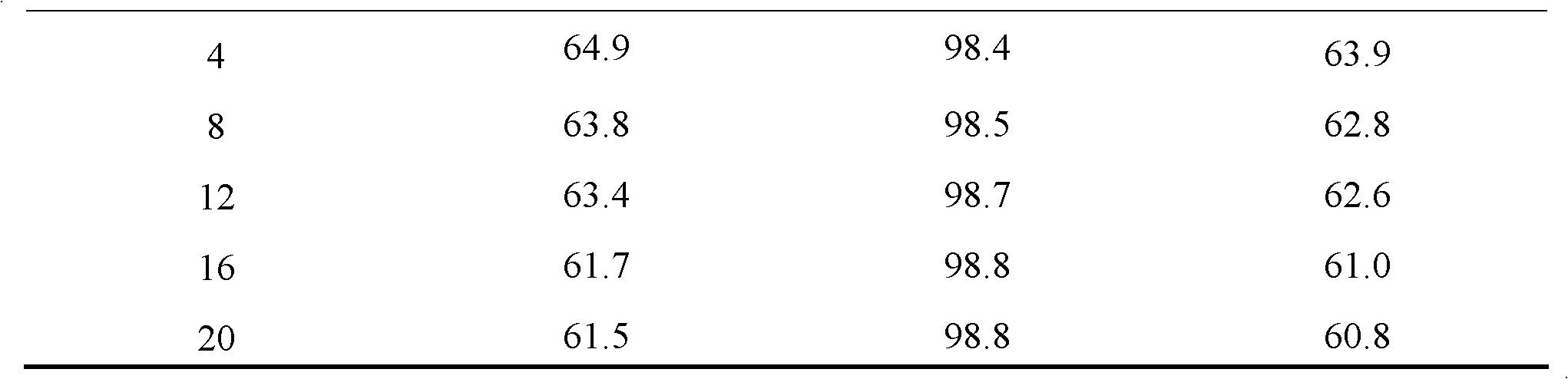
[0023] The composition of catalyst A is 0.375% Pt, 0.5% Au, 0.5% Sn, 1.4% K, 1.0% Cl and 0.2% S supported on γ-Al 2 o 3 superior. The preparation method is as follows:
[0024] (1) 0.5% Sn / Al 2 o 3 preparation of
[0025] A certain amount of carrier is placed in the dipping bottle, and SnCl equivalent to 0.5% Sn content will be prepared 2 The hydrochloric acid solution (equivalent to 2% of the weight of the alumina carrier) impregnating liquid is quickly put into the carrier, kept for 4 hours, and constantly shaken to make the impregnating evenly, and then the remaining impregnating liquid is poured out, taken out and dried, at 60°C and 120°C Bake for 4 hours each, and then pass air into the tubular furnace (SV=5000h -1 ), roasting at 550°C for 4 hours; then switch the air through a constant temperature water bath at 70°C, and treat with steam for 4 hours.
[0026] (2) 0.375%Pt~0.5%Au~0.5%Sn / Al 2 o 3 preparation of
[0027] 0.5% Sn / Al 2 o 3 Placed in the dipping bo...
Embodiment 2
[0038] The composition of catalyst B is 0.5% Pt, 0.5% Au, 0.5% Sn, 1.4% Li, 1.0% Cl and 0.2% S supported on γ-Al 2 o 3 superior. The preparation method is as follows:
[0039] (1) 0.5% Sn / Al 2 o 3 preparation of
[0040] A certain amount of carrier is placed in the dipping bottle, and SnCl equivalent to 0.5% Sn content will be prepared 2 The hydrochloric acid solution (equivalent to 2% of the weight of the alumina carrier) impregnating liquid is quickly put into the carrier, kept for 4 hours, and constantly shaken to make the impregnating evenly, and then the remaining impregnating liquid is poured out, taken out and dried, at 60°C and 120°C Bake for 4 hours each, and then pass air into the tubular furnace (SV=5000h -1 ), roasting at 550°C for 4 hours; then switch the air through a constant temperature water bath at 70°C, and treat with steam for 4 hours.
[0041] (2) 0.5%Pt~0.5%Au~0.5%Sn / Al 2 o 3 preparation of
[0042] 0.5% Sn / Al 2 o 3 Placed in the dipping bottl...
Embodiment 3
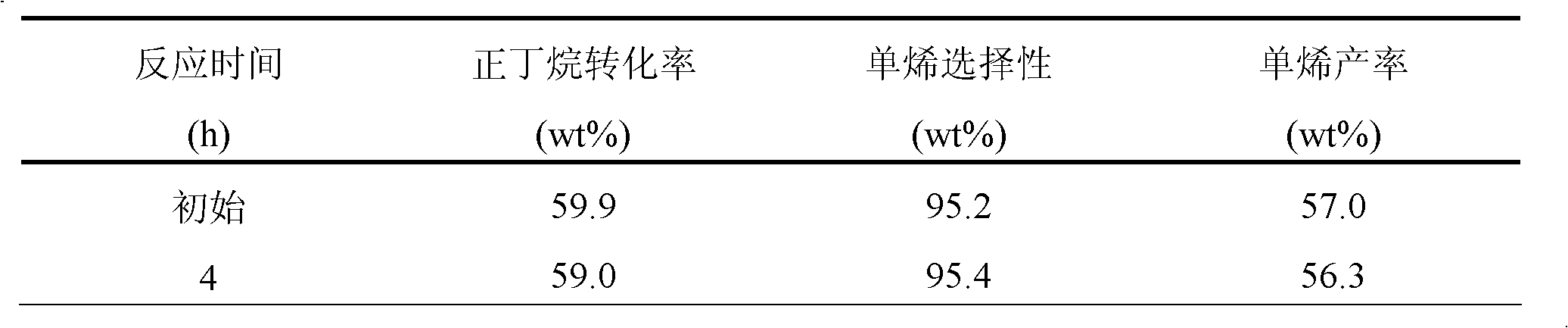
[0054] Catalyst C is composed of 0.375% Ru, 0.5% Au, 0.5% Sn, 1.4% K, 1.0% Cl and 0.2% S supported on ZSM-5 molecular sieve. The preparation method is the same as in Example 1, with H 2 PtCl 6 Change to RuCl 3 hydrochloric acid solution, the carrier was changed to ZSM-5. After elemental analysis, it can be seen that the actual load is consistent with the theoretical load.
[0055] The reaction performance of this catalyst is as shown in table 3:
[0056] Table 3. Propane dehydrogenation reaction performance
[0057]
[0058] Reaction conditions: reaction temperature 620°C, LHSV=3h -1 , H 2 / C 3 (mol / mol)=1, the reaction pressure is 1 atm.
[0059] The catalyst of this composition deactivates faster.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com