Preparation method of single-ionic conductor SiO2@Li<+1> with core-shell structure in polymer electrolyte
A core-shell structure and electrolyte technology, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of increasing the internal resistance of the battery, affecting the battery performance, and the migration number cannot be significantly improved, and achieving good dispersion. and morphology, excellent electrochemical properties, improved mechanical properties and ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
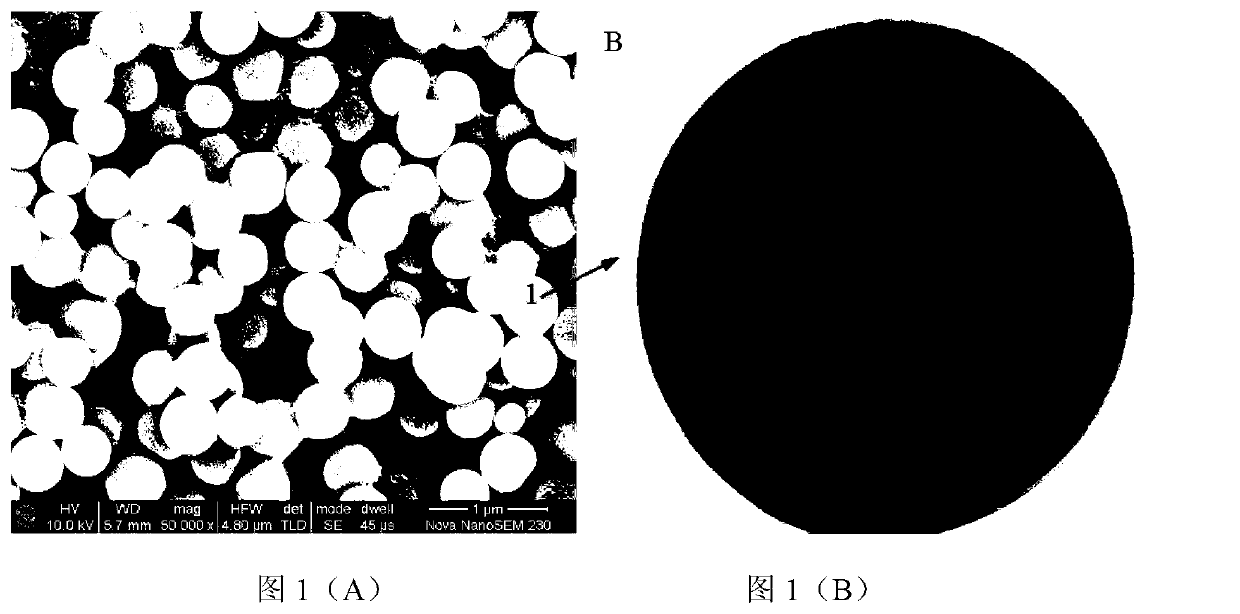
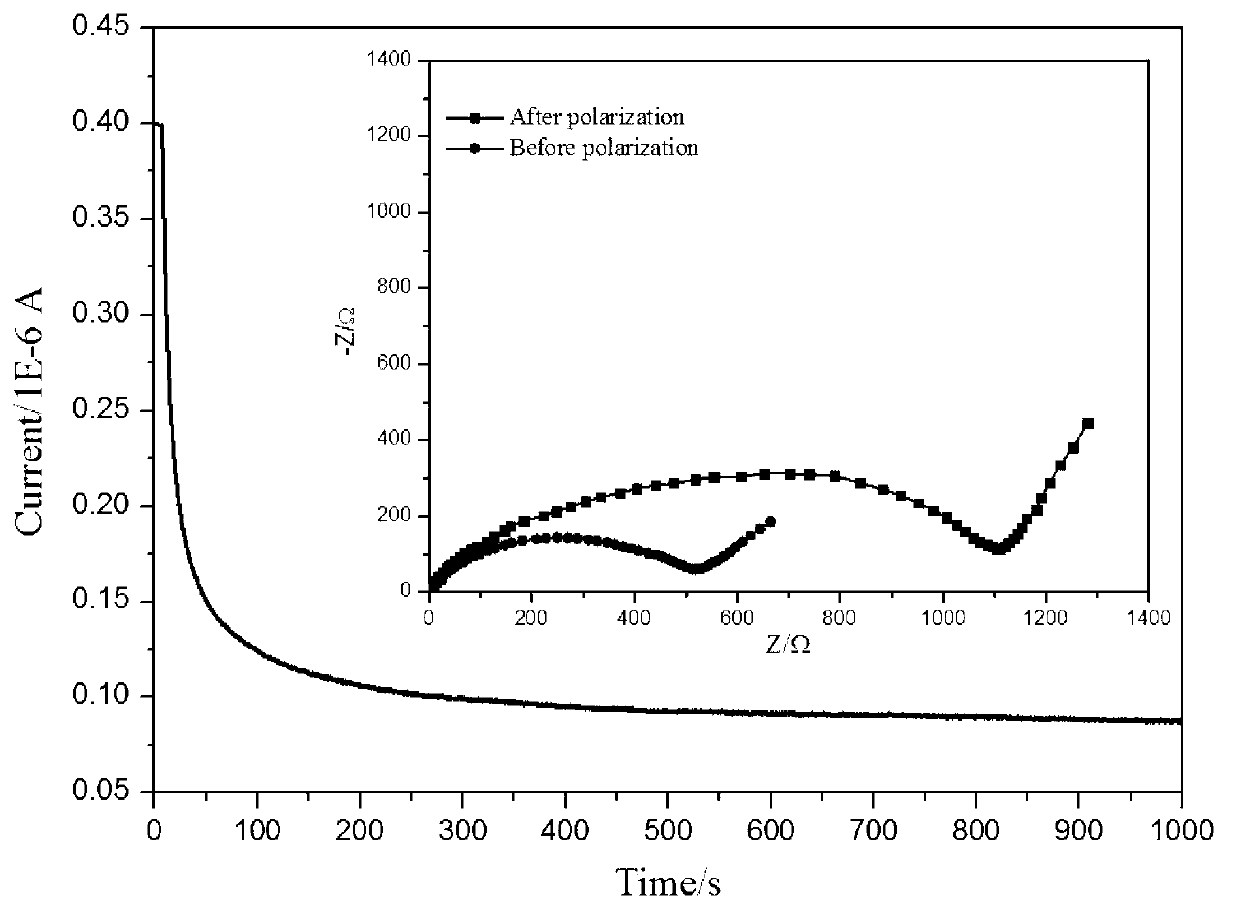
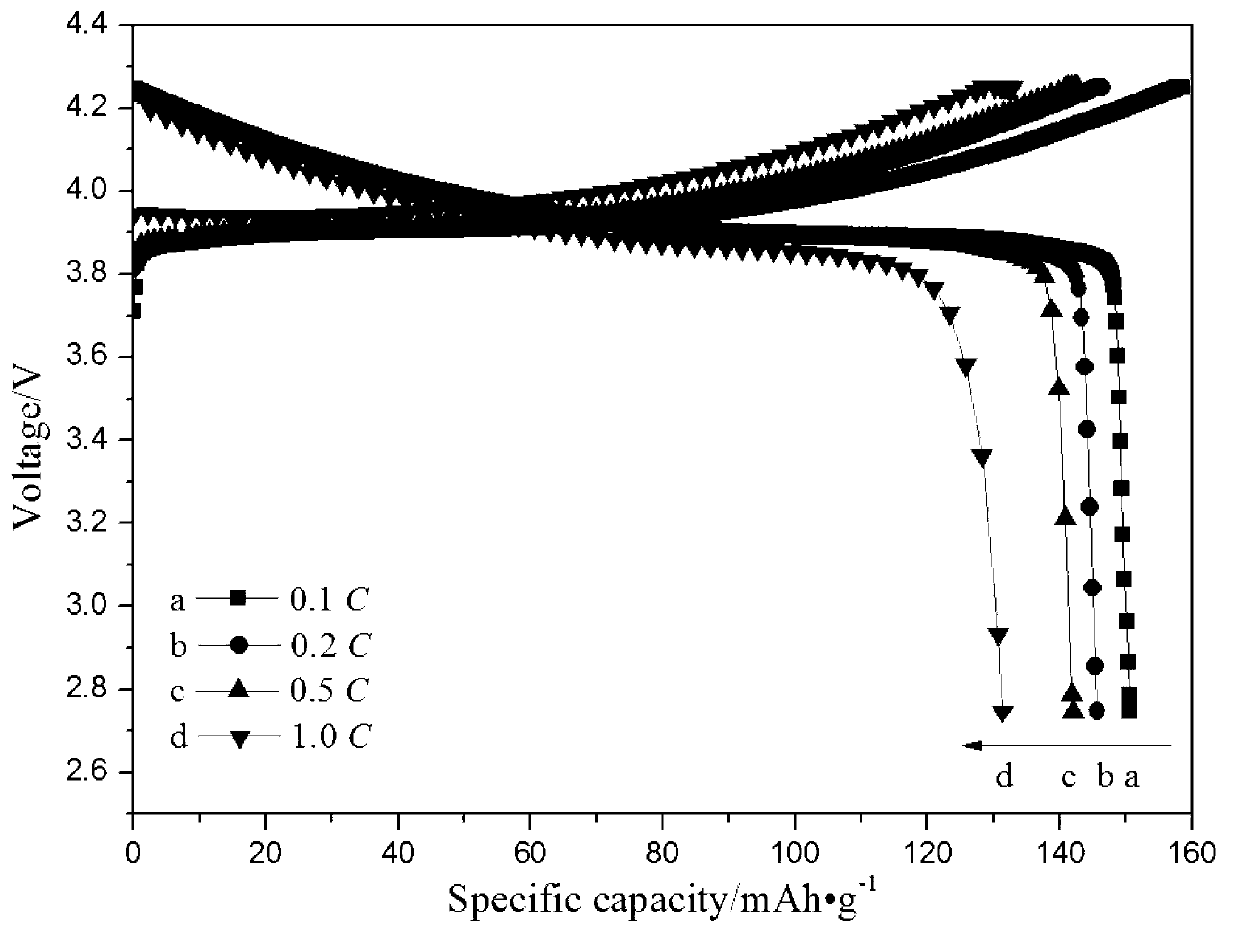
[0024] Use vinyltrimethylsilane as the silicon source, in the ethanol solution of water, choose ammonia water as the catalyst, control m (silicon source): m (water): m (catalyst): m (absolute ethanol) = 1.5: 2.5 : 1: 25. At 40°C, after hydrolysis for 2h, 4h, 6h, 8h and 10h, respectively, filter or centrifuge to prepare monodisperse spherical SiO 2 , and then freely polymerized with sodium p-styrenesulfonate at 80 °C for 12 h in the presence of azobisisobutyronitrile to obtain SiO 2 Na + , and finally ion-exchange SiO2Na+ and lithium hydroxide at 90°C for 20 hours, and the product is vacuum-dried at 60°C to obtain SiO 2 Li + . Weigh the prepared 0.4g SiO 2 Li + Uniformly dispersed in N,N-dimethylformamide (DMF) solution of polyvinylidene fluoride and hexafluoropropylene copolymer (PVDF-HFP) under ultrasonic vibration, obtained after vigorous stirring at 40°C for 4h The uniform and transparent gel was left to stand for 30 minutes, then vacuum degassed, and cast on a polyte...
Embodiment 2
[0029] Using the mixed solution of vinyltris(β-methylethoxy)silane and butyl orthosilicate as the silicon source, in the ethanol solution of water, choose the mixed solution of ammonia water and sodium acetate as the catalyst, and control m (silicon source): m (water): m (catalyst): m (absolute ethanol) = 1.5: 2.5: 1: 25. Hydrolyze at 40°C for 8 hours, filter or centrifuge to prepare monodisperse spherical SiO2, and then in azobisisobutyronitrile Free polymerization with sodium p-styrene sulfonate at 80°C for 12h in the presence of SiO 2 Na + , and finally the SiO 2 Na + Ion exchange with lithium hydroxide at 90°C for 20 hours, and the product is vacuum dried at 60°C to obtain SiO 2 Li + . Disperse the prepared 0.4g SiO2Li+ uniformly in the N,N-dimethylformamide (DMF) solution of polyvinylidene fluoride and hexafluoropropylene copolymer (PVDF-HFP) under ultrasonic vibration, and forcefully The homogeneous and transparent gel obtained after stirring for 4 hours was left t...
Embodiment 3
[0033] Use vinyl tris(β-methylethoxy)silane as the silicon source, in the ethanol solution of water, choose the mixed solution of ammonia water and sodium hydroxide as the catalyst, and control m (silicon source): m (water): m (Catalyst): m (absolute ethanol) = 1.5: 2.5: 1: 25. Hydrolyze at 40°C for 8 hours, filter or centrifuge to prepare monodisperse spherical SiO2, and then in the presence of azobisisobutyronitrile Sodium styrene sulfonate was freely polymerized at 80°C for 8h, 10h, 12h and 14h to obtain SiO 2 Na + , and finally the SiO 2 Na + Ion exchange with lithium hydroxide at 90°C for 16h, 18h, 20h and 24h, and the product is vacuum dried at 60°C to obtain SiO 2 Li + . Disperse the prepared 0.4g SiO2Li+ uniformly in the N,N-dimethylformamide (DMF) solution of polyvinylidene fluoride and hexafluoropropylene copolymer (PVDF-HFP) under ultrasonic vibration, and forcefully The homogeneous and transparent gel obtained after stirring for 4 hours was left to stand for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com