Tebufenpyrad/metaflumizone wettable powder and production method thereof
The technology of wettable powder and cyflufenazone is applied in the field of wettable powders of fenflume and fenflufen, which can solve the problems such as documents that do not find the compound and application of fenflunom and fenflufen, and the like, Achieve the effect of overcoming and pest resistance, reducing pollution, and prolonging control time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
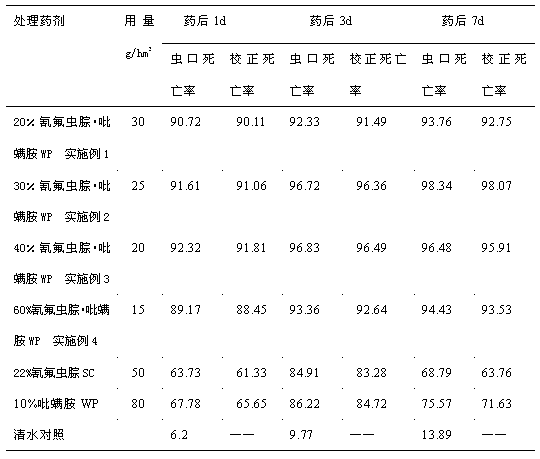
Examples
Embodiment 1
[0020] The wettable powder of tebufenfen and metaflumizone in this embodiment consists of the following components in weight percentage:
[0021] Tebufenfen 10%
[0022] Metaflumizone 10%
[0023] Wetting agent 1.5%
[0024] Dispersant 5%
[0025] Carrier balance
[0026] In this embodiment, the weight content of the active ingredients tebufenfen and metaflumizone is 20%. The wetting agent adopts pull-off powder; the dispersant adopts sodium lignosulfonate and methyl naphthalene sulfonate formaldehyde condensate sulfate, and the weight content is 3% and 2% respectively; the carrier adopts sodium sulfate.
[0027] The production method of the wettable powder of tebufenfen and metaflumizone in this embodiment is to add the active ingredients tebufenfen and metaflumizone into the carrier, then add a wetting agent and a dispersant, mix evenly, and After pulverized by the jet mill, mix it evenly with a mixer.
Embodiment 2
[0029] The difference between this example and Example 1 is that the wettable powder of tebufenfen and metaflumizone in this example consists of the following components by weight percentage:
[0030] Tebufenfen 10%
[0031] Metaflumizone 20%
[0032] Wetting agent 2%
[0033] Dispersant 4%
[0034] Carrier balance
[0035] The weight content of the active ingredients tebufenfen and metaflumizone in this example is 30%. The wetting agent adopts sodium lauryl sulfate; the dispersant adopts polycarboxylate; the carrier adopts white sugar and soluble starch, and the weight content of white sugar is 20%.
Embodiment 3
[0037] The difference between this example and Example 1 is that the wettable powder of tebufenfen and metaflumizone in this example consists of the following components by weight percentage:
[0038] Tebufenfen 15%
[0039] Metaflumizone 25%
[0040] Wetting agent 2%
[0041] Dispersant 5%
[0042] Carrier balance
[0043] In this embodiment, the weight content of the active ingredients tebufenfen and metaflumizone is 40%. The wetting agent is sodium lauryl sulfate; the dispersant is sodium N-methyl fatty acyl taurate; the carrier is white carbon black.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com