Method for removing trace olefin from aromatic hydrocarbon
An aromatics and removal technology, applied in the direction of chemical change purification/separation, organic chemistry, etc., can solve the problems of poor catalyst activity stability, large internal diffusion resistance, large catalyst coking deactivation rate, etc. Simple process, avoids reaction and regenerates the effect of frequent switching operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of B-AlPO 4 -5 / Hβ core-shell composite molecular sieve catalyst, denoted as CAT-1.
[0033] 80 grams of Hβ microporous molecular sieve powder was used as the inner core, and stirred and mixed with 500 grams of deionized water for 1.0 hour to obtain a mixture A. According to Al 2 o 3 :P 2 o 5 :B 2 o 3 :OXAA:H 2 O: ETHA: TEA raw material molar ratio is 1: 0.432: 0.191: 0.304: 25.368: 2.352: 0.756, 40 grams of monohydrate alumina, and the corresponding amount of phosphoric acid (H 3 PO 4 , purity 85wt%), boric acid, oxalic acid (OXAA), deionized water, absolute ethanol (ETHA) and triethylamine (TEA) were stirred and mixed for 0.5 hours to prepare mixture B. Add mixture A to mixture B, continue stirring and mixing for 4.5 hours, place in a stainless steel reactor, and crystallize at 150°C for 3 days; then filter, wash with water, dry, and roast at 550°C for 5 hours to remove the template agent to obtain the inner core Hβ B-AlPO with microporous molecular...
Embodiment 2
[0035] Synthesis of Mg-AlPO 4 -5 / Hβ composite molecular sieve catalyst, denoted as CAT-2.
[0036] 2.0 g of Hβ microporous molecular sieve powder was used as the inner core, and stirred and mixed with 20 g of deionized water for 1.0 hour to obtain a mixture A. According to Al 2 o 3 :P 2 o 5 :MgO:OXAA:H 2 O: ETHA: TPA raw material molar ratio 1: 0.541: 0.227: 0.061: 27.482: 2.353: 0.758, 40 grams of monohydrate alumina, and the corresponding amount of phosphoric acid (H 3 PO 4 , 85Wt%), magnesium acetate, oxalic acid (OXAA), deionized water, ethanol (ETHA), and tri-n-propylamine (TPA) were stirred and mixed for 0.5 hours to prepare mixture B. Add mixture A to mixture B, continue stirring and mixing for 4.5 hours, place in a stainless steel reactor, and crystallize at 150°C for 3 days; then filter, wash with water, dry, and roast at 550°C for 5 hours to remove the template agent to obtain the inner core Hβ Mg-AlPO with microporous molecular sieve mass accounting for 1.3%...
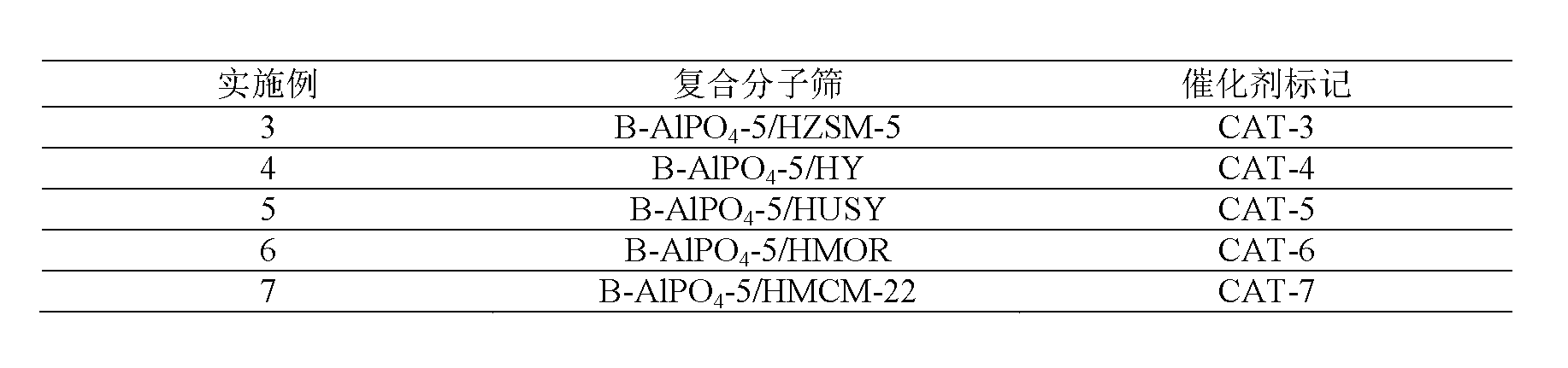
Embodiment 3~7
[0038] HZSM-5 molecular sieve, HY molecular sieve, HUSY molecular sieve, HMOR molecular sieve, HMCM-22 molecular sieve were used as core microporous molecular sieves respectively, and after hydrothermal synthesis, B-AlPO with core microporous molecular sieves accounting for 34.8% was obtained. 4 The core-shell type composite molecular sieve catalyst of -5 / microporous molecular sieve, other operations are the same as in Example 1, and the results are listed in Table 1.
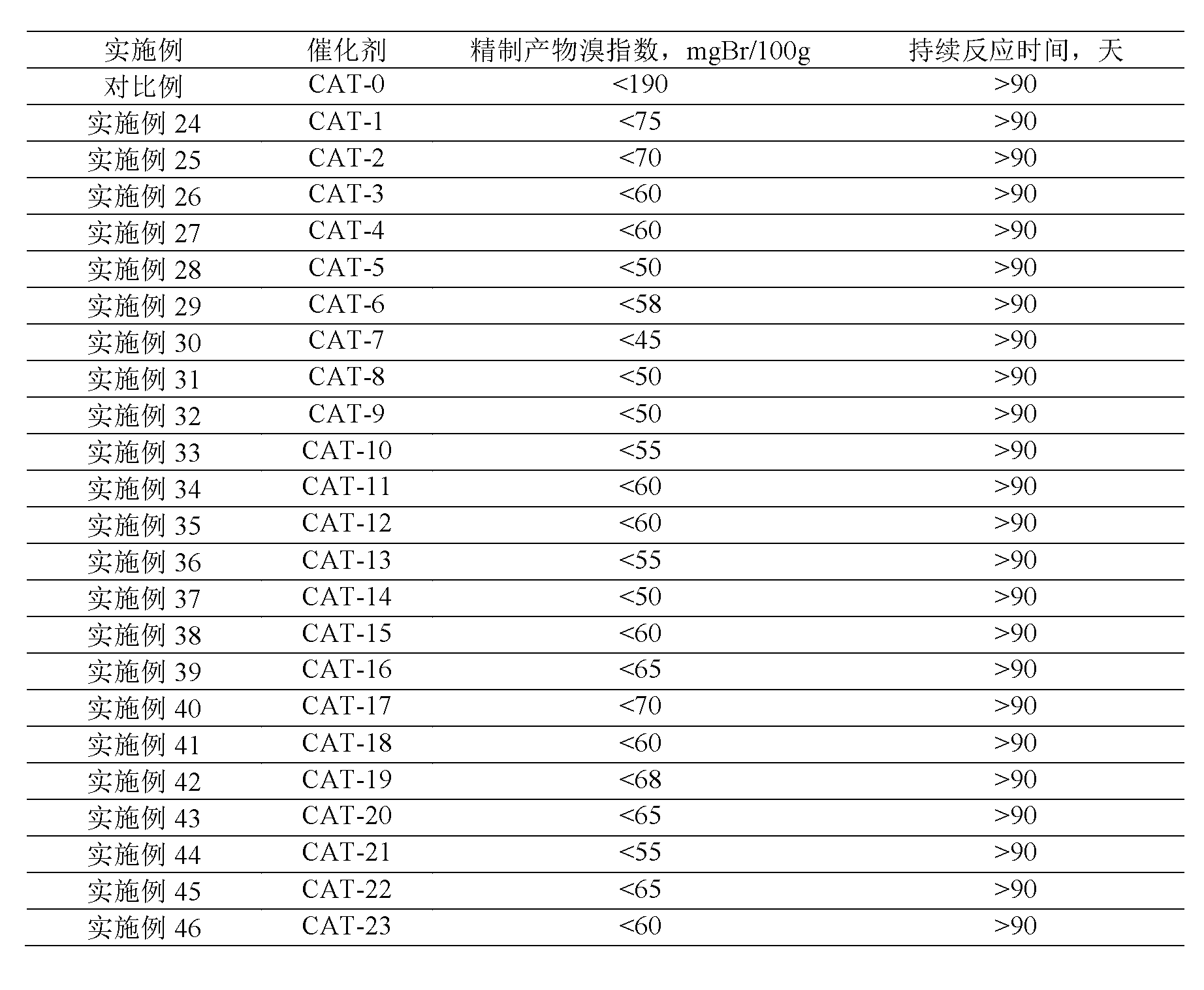
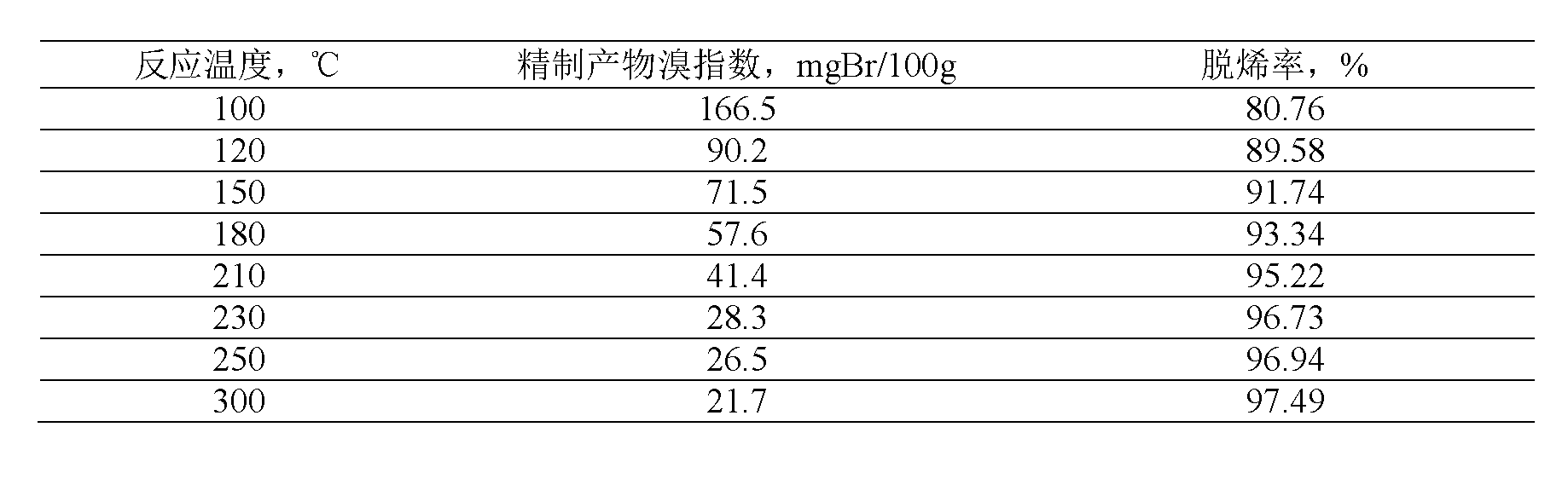
[0039] Table 1
[0040]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Index | aaaaa | aaaaa |
| Index | aaaaa | aaaaa |
| Index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com