Preparation method of n-butyl benzoate
A technology of n-butyl benzoate and a manufacturing method, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as difficulty in heating, potential safety hazards, prolonged reaction time, etc., and achieves simple and convenient operation process. The effect of stability, safe transportation and storage, and less pollution of the three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
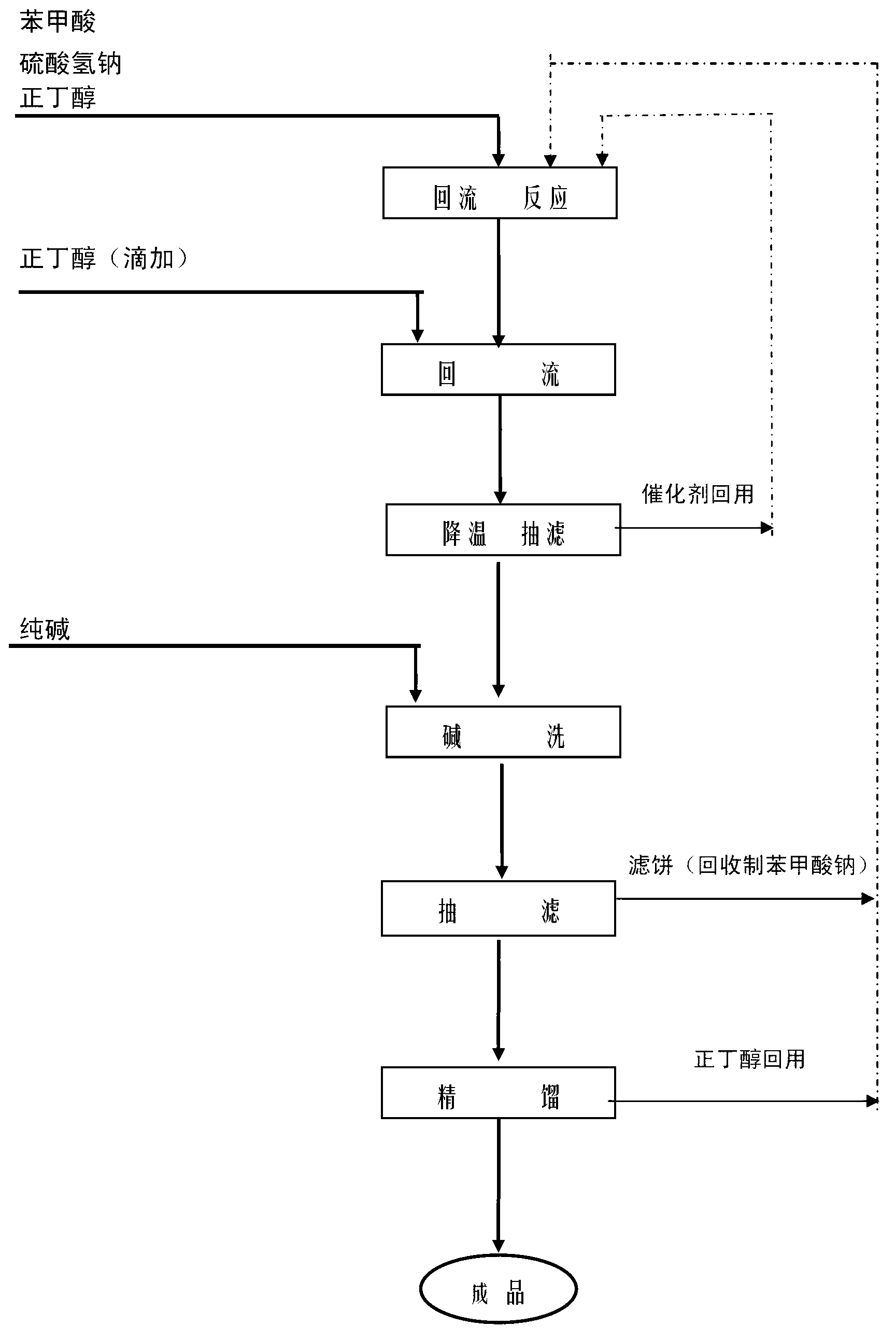
[0028] Add 450g of benzoic acid, 170g of n-butanol, and 25.4g of sodium bisulfate into a 1000ml four-necked bottle, install a water separator, a reflux condenser and a thermometer, stir and heat to reflux to separate water. After the reaction temperature rises to 135°C, add 170g of n-butanol dropwise. When the temperature rises to 150°C and no water comes out of the water separator, stop the reaction. The reaction time is 1.5h. After the reaction, the temperature was lowered to below 40°C to recover the catalyst by suction filtration and 722g of the filtrate was obtained. Sample analysis showed that the content of n-butyl benzoate was 88.75% (GC), the content of benzoic acid was 0.37% (GC), and the content of n-butanol was 10.1% (GC). . The filtrate was neutralized by adding 20g of solid alkali at 60°C for 20 minutes, and then suction filtered again. The crude product was sampled and analyzed, and the content of benzoic acid (GC) was 0.016%. After rectification, 610 g of n-bu...
Embodiment 2
[0030] Feeding amount and reaction operation are the same as in Example 1. After the reaction is completed, the catalyst is recovered by cooling and suction filtration and 724g of filtrate is obtained. Sampling analysis shows that the content of n-butyl benzoate is 89.29% (GC), the content of benzoic acid is 0.46% (GC), and n-butanol Content 9.55% (GC). The filtrate was neutralized by adding 10 g of solid alkali at 60°C for 60 minutes, and then suction filtered again. The crude product was sampled and analyzed, and the content of benzoic acid (GC) was 0.17%. After rectification, 614g of n-butyl benzoate was obtained. Sample analysis showed that the content was 99.4% (GC), the acid value was 1.48 (mgKOH / g), and the total yield was 93.4%.
Embodiment 3
[0032] Feeding amount and reaction operation are the same as in Example 1. After the reaction is over, the material is distilled under reduced pressure to recover n-butanol, and the remaining material is 670g. Sampling analysis shows that the content of n-butyl benzoate is 96.42% (GC), and the content of benzoic acid is 0.47% (GC). The n-butanol content is 2.61% (GC). Then add 20g of solid alkali to neutralize at 60°C for 20 minutes, and then filter again. The crude product is sampled and analyzed. The content of n-butyl benzoate is 97% (GC), the content of benzoic acid is 0.33% (GC), and the content of n-butanol is 2.18% (GC). .
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com