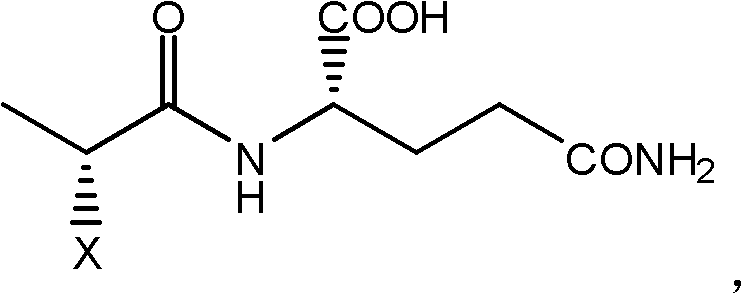
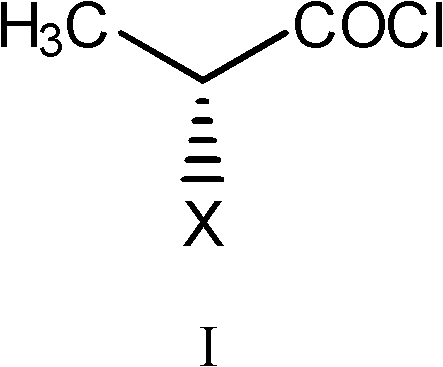Method for preparing D-2-substituted propionyl-L-glutamine
A technology of glutamine and D-2-, applied in the field of preparing D-2-substituted propionyl-L-glutamine, can solve the problem of unstable product conversion rate, low conversion rate, D-2-substituted propionyl chloride The problem of increasing consumption, etc., achieves the effect of being conducive to large-scale industrial production, stable consumption and conversion rate, and clear consumption trend.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
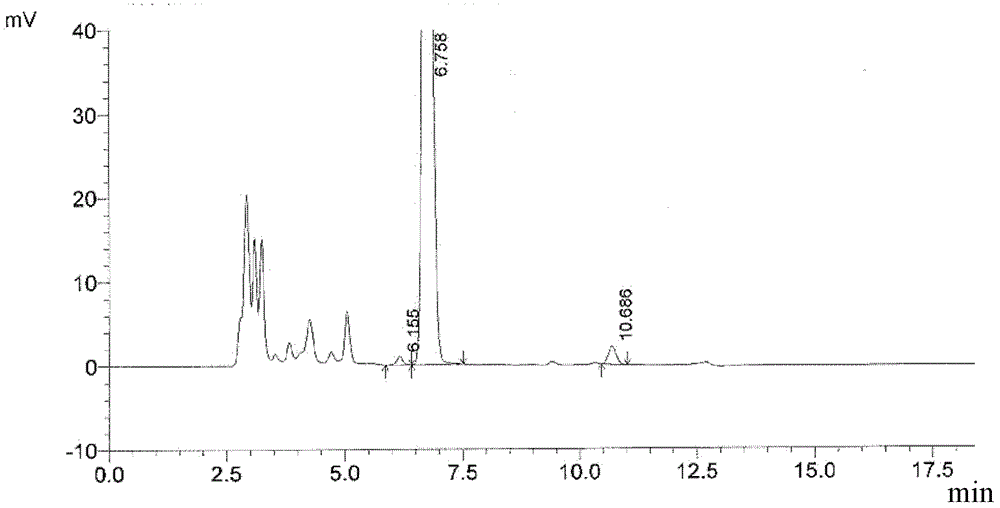
Image
Examples
Embodiment 1
[0030] Add L-glutamine (60g), water (120g) and toluene (120mL) into the reaction flask, cool down to 0±2°C, add dropwise 5mol / L sodium hydroxide solution (102g), dissolve and clarify, add four Butylammonium bromide (3g) and sodium chloride (36g) were stirred evenly. Slowly add D-2-chloropropionyl chloride (62.6g) diluted with toluene (62.6mL) dropwise. After adjusting the pH to 10, start to drop 5mol / L sodium hydroxide solution (150g), and control the temperature at 0±2°C. pH value 9-10, reaction 2h. The layers were allowed to stand, the water layer was taken, concentrated hydrochloric acid (49.7g) was added dropwise to adjust the pH to 2.0, and crystallized. Suction filtration, suction drying, vacuum drying. 94.0 g of white or off-white solid was obtained. The yield is 96.7% (calculated as L-glutamine).
Embodiment 2
[0032] Add L-glutamine (100g), water (250g), petroleum ether (60-90°C, 300mL) into the reaction flask, cool down to 0±3°C, add dropwise 4.5mol / L sodium hydroxide solution (190g ), dissolved and clarified, added tetrabutylammonium chloride (2g), sodium iodide (70g) and stirred evenly. Slowly add D-2-methanesulfonyloxypropionyl chloride (146g) diluted with petroleum ether (60-90°C, 219mL) dropwise, after adjusting the pH to 10, start to drop 5mol / L sodium hydroxide solution (244g), Control the temperature at 0±3°C, pH 9-10, and react for 3 hours. The layers were allowed to stand, and the water layer was taken, and concentrated hydrochloric acid (82.8 g) was added dropwise to adjust the pH value to 1.6, and crystallized. Suction filtration, suction drying, vacuum drying. 197.5 g of white or off-white solid was obtained. The yield is 97.5% (calculated as L-glutamine). 1H-NMR (400MHz, DMSO-d6) 12.63(s, 1H), 8.63(s, 1H), 7.40(s, 1H), 6.90(d, 1H), 5.13(q, 1H), 4.15-4.25(m , 1H),...
Embodiment 3
[0034] Add L-glutamine (80g), water (144g) and ethyl acetate (120mL) into the reaction flask, cool down to 0±3°C, add dropwise 5mol / L sodium hydroxide solution (144g), dissolve and clarify, Add tetrabutylammonium bisulfate (6.4g) and potassium chloride (56g) and stir well. Slowly add D-2-bromopropionyl chloride (108g) diluted with ethyl acetate (108mL) dropwise, adjust the pH to 10, then start to drop 4mol / L sodium hydroxide solution (208g), control the temperature at 0±3°C, pH value 9-10, reaction 4h. The layers were allowed to stand, the water layer was taken, concentrated hydrochloric acid (66.2 g) was added dropwise to adjust the pH to 1.8, and crystallized. Suction filtration, suction drying, vacuum drying. 147.5 g of white or off-white solid was obtained. The yield is 96.2% (calculated as L-glutamine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com