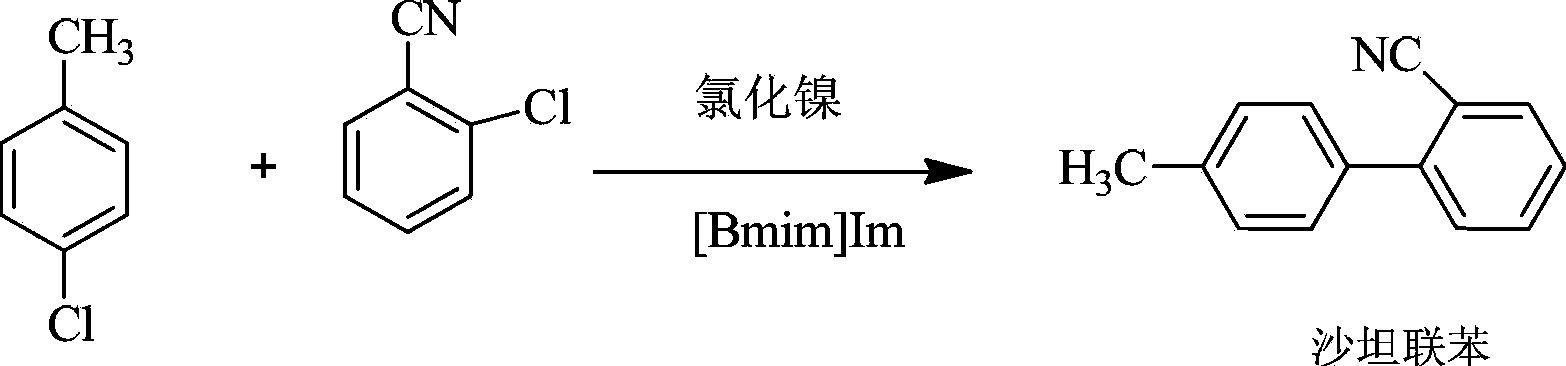
The preparation method of sartan biphenyl
A technology of sartan biphenyl and o-chlorobenzonitrile, which is applied in the field of preparation of sartan biphenyl, can solve problems such as unfavorable continuous large-scale industrial production, difficult recovery of catalysts, harsh reaction conditions, etc., and achieve great implementation value and social Economic benefits, low production costs, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 2 mmol of [Bmim] Im in 250 mL of purified water, mix well, add 0.13 g of nickel chloride (0.001 mol), and stir at 23±3°C for 30 min. In flask, add 13.76g (0.1mol) o-chlorobenzonitrile, 13.92g (0.11mol) p-chlorotoluene, stir 10min at 20 ℃, then the above-mentioned [Bmim]Im ionic liquid loaded with nickel chloride catalyst is added to In the mixed solution of o-chlorobenzonitrile and p-chlorobenzonitrile, stir and react at 25±3°C for 3h, then let stand for 0.5h. 19.0 g (yield: 98.45%) of a white solid was obtained by filtration with a purity of 99.4%. Mp: 48~49℃, 1 HNMR (500MHz, CDCl 3 ): (δppm): 2.45 (s, 3H), 7.25-7.35 (t, 2H), 7.41-7.58 (m, 4H), 7.61-7.82 (m, 2H).
Embodiment 2
[0025] Dissolve 2 mmol of [Bmim] Im in 300 mL of purified water, mix well, add 1.3 mg of nickel chloride (0.01 mmol), and stir at 25±3°C for 25 min. In flask, add 13.78g (0.1mol) o-chlorobenzonitrile, 13.94g (0.11mol) p-chlorotoluene, stir 15min at 20 ℃, then the above-mentioned [Bmim]Im ionic liquid loaded with nickel chloride catalyst is added to In the mixed solution of o-chlorobenzonitrile and p-chlorobenzonitrile, stir and react at 27±3°C for 5h, then let stand for 0.5h. 19.11 g (yield: 99.02%) of a white solid was obtained by filtration with a purity of 99.5%. Mp and 1 HNMR is the same as in Example 1.
Embodiment 3
[0027] Dissolve 2 mmol of [Bmim] Im in 250 mL of purified water, mix well, add 0.13 g of nickel chloride (0.001 mol), and stir at 24±3°C for 30 min. In flask, add 13.78g (0.1mol) o-chlorobenzonitrile, 15.19g (0.12mol) p-chlorotoluene, stir 15min at 22 ℃, then the above-mentioned [Bmim]Im ionic liquid loaded with nickel chloride catalyst is added to In the mixed solution of o-chlorobenzonitrile and p-chlorobenzonitrile, stir and react at 25±3°C for 6.5h, then let stand for 0.5h. 19.06 g (yield: 98.76%) of a white solid was obtained by filtration with a purity of 99.1%. Mp and 1HNMR are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com