Preparation method of 6-chloro-1,3-dimethyluracil
A technology of dimethyl uracil and dimethyl barbituric acid is applied in the field of preparation of 6-chloro-1,3-dimethyl uracil, and can solve the problem of large dosage of phosphorus oxychloride and high requirements for reaction equipment , increase the difficulty of operation and other problems, to achieve the effect of simplifying post-processing, easy operation, and reducing the generation of waste acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The invention provides a preparation method of 6-chloro-1,3-dimethyluracil, comprising the following steps:
[0037] A) The 1,3-dimethylbarbituric acid and the chlorinating agent are refluxed in a water-immiscible organic solvent to obtain a reaction mixture; the chlorinating agent includes phosphorus oxychloride;
[0038] B) After adding water to the reaction mixture to quench the reaction, 6-chloro-1,3-dimethyluracil is obtained.
[0039] The present invention uses 1,3-dimethylbarbituric acid and a chlorinating agent including phosphorus oxychloride as raw materials, reacts in an organic solvent immiscible with water, and can obtain 6-chlorobarbiturate after quenching the reaction -1,3-Dimethyluracil. Further, in the process of reacting 1,3-dimethylbarbituric acid and a chlorinating agent comprising phosphorus oxychloride, the present invention uses a water-immiscible organic solvent as the reaction medium, which can greatly reduce the three The consumption of phosp...
Embodiment 1
[0061] In a 1000L dry reactor, add 440kg of methanol, 44kg of 1,3-dimethylurea and 26kg of sodium methoxide, and start stirring. After 0.5 hours, slowly add 66kg of dimethyl malonate. After the addition is complete, the temperature is raised for reflux reaction , after reacting for 6 hours, down to room temperature, centrifuged to obtain 88kg1,3-dimethyl barbituric acid sodium salt; in the obtained 1,3-dimethyl barbituric acid sodium salt, add 100kg water, and then use 30% concentrated hydrochloric acid to adjust the pH value of the mixture of 1,3-dimethylbarbituric acid sodium salt and water to 1~2, and finally centrifuged to obtain 58.5kg of 1,3-dimethylbarbituric acid product , and the yield was 75%.
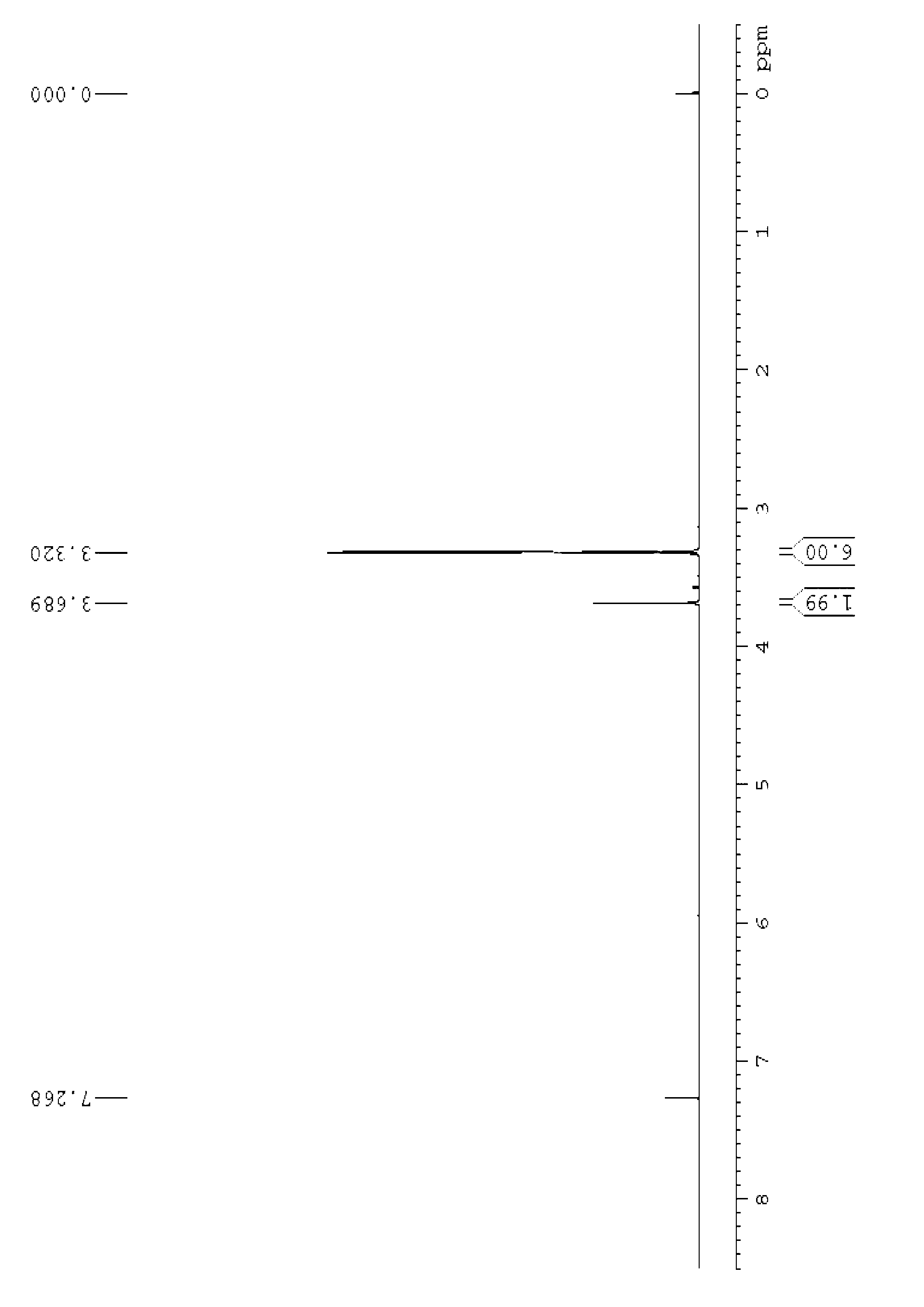
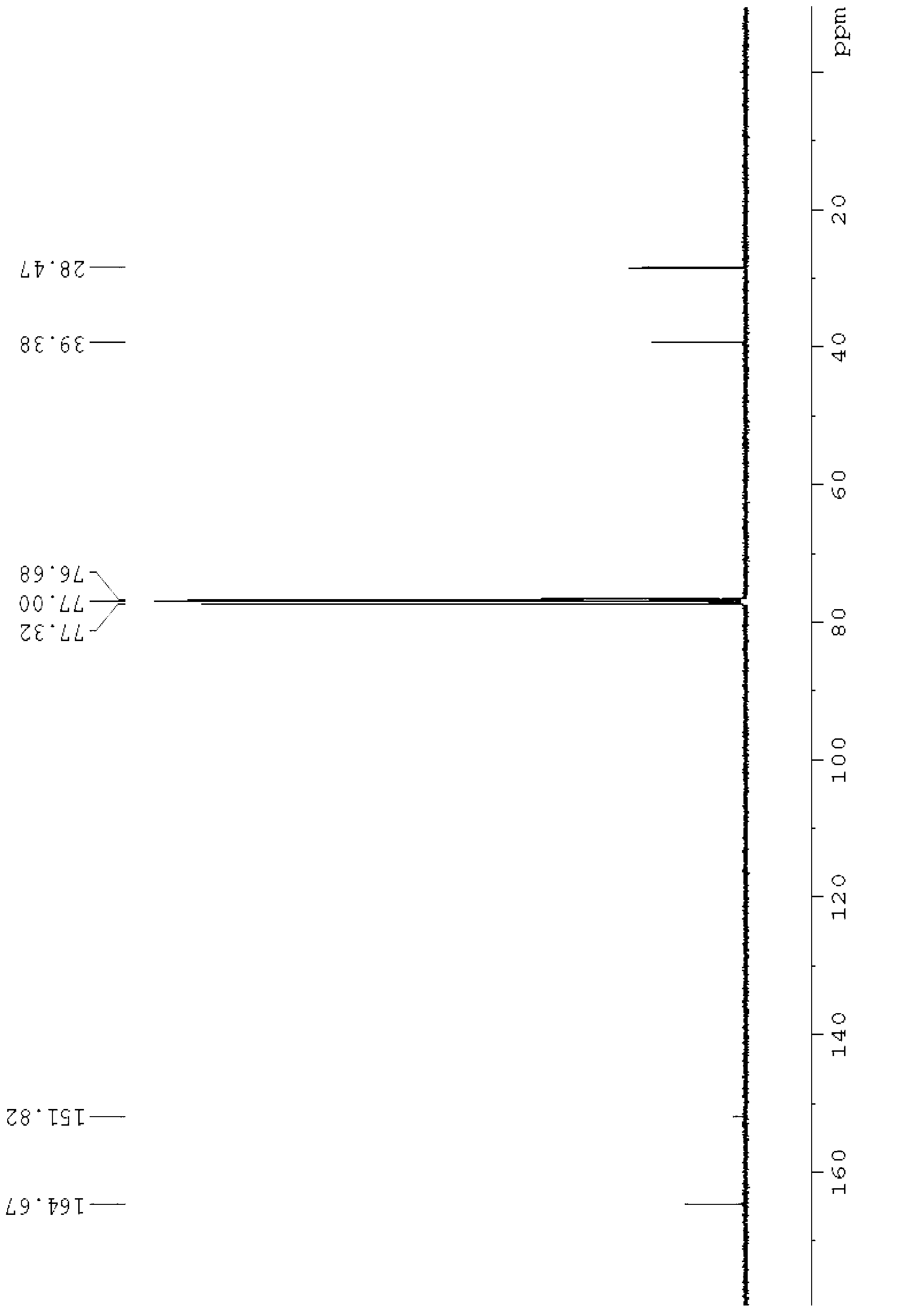
[0062] NMR analysis of 1,3-dimethylbarbituric acid, see figure 1 and figure 2 , figure 1 It is the 1,3-dimethylbarbituric acid hydrogen nuclear magnetic resonance spectrogram prepared in the embodiment 1 of the present invention, figure 2 It is the carbon nuclear magnet...
Embodiment 2
[0064] In a 100L dry reaction kettle, add 50kg of xylene, 10kg of 1,3-dimethylbarbituric acid prepared in Example 1 and 12kg of phosphorus oxychloride, start stirring, and cool the above mixture to -5~0 ℃, under the condition of less than 10℃, slowly add 5kg of methanol dropwise, after the dropwise addition, slowly raise the temperature for reflux reaction, after 5 hours of reaction, stop heating, lower to room temperature, and slowly add 12kg of methanol to it under the temperature below 40℃ After adding water, continue to stir for 0.5 hours, cool down to room temperature, centrifuge to obtain solid crude product and centrifuged mother liquor, add 35kg methanol and 0.5kg activated carbon to the crude product, decolorize through reflux, filter, cool and crystallize, centrifuge, and dry to obtain 10.06 kg fine 6-chloro-1,3-dimethyluracil; the centrifuged mother liquor was layered, washed three times with 30kg of water, and then dried with 0.5kg of anhydrous sodium sulfate,
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com