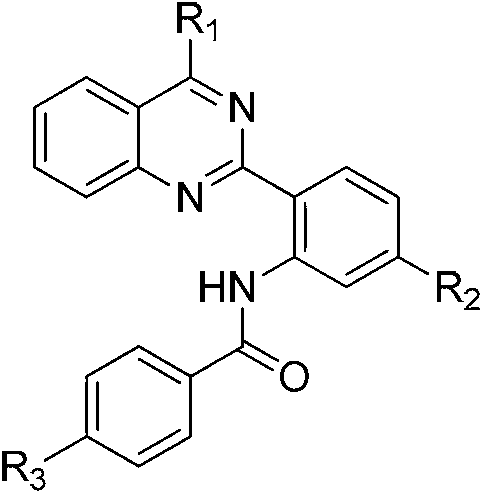
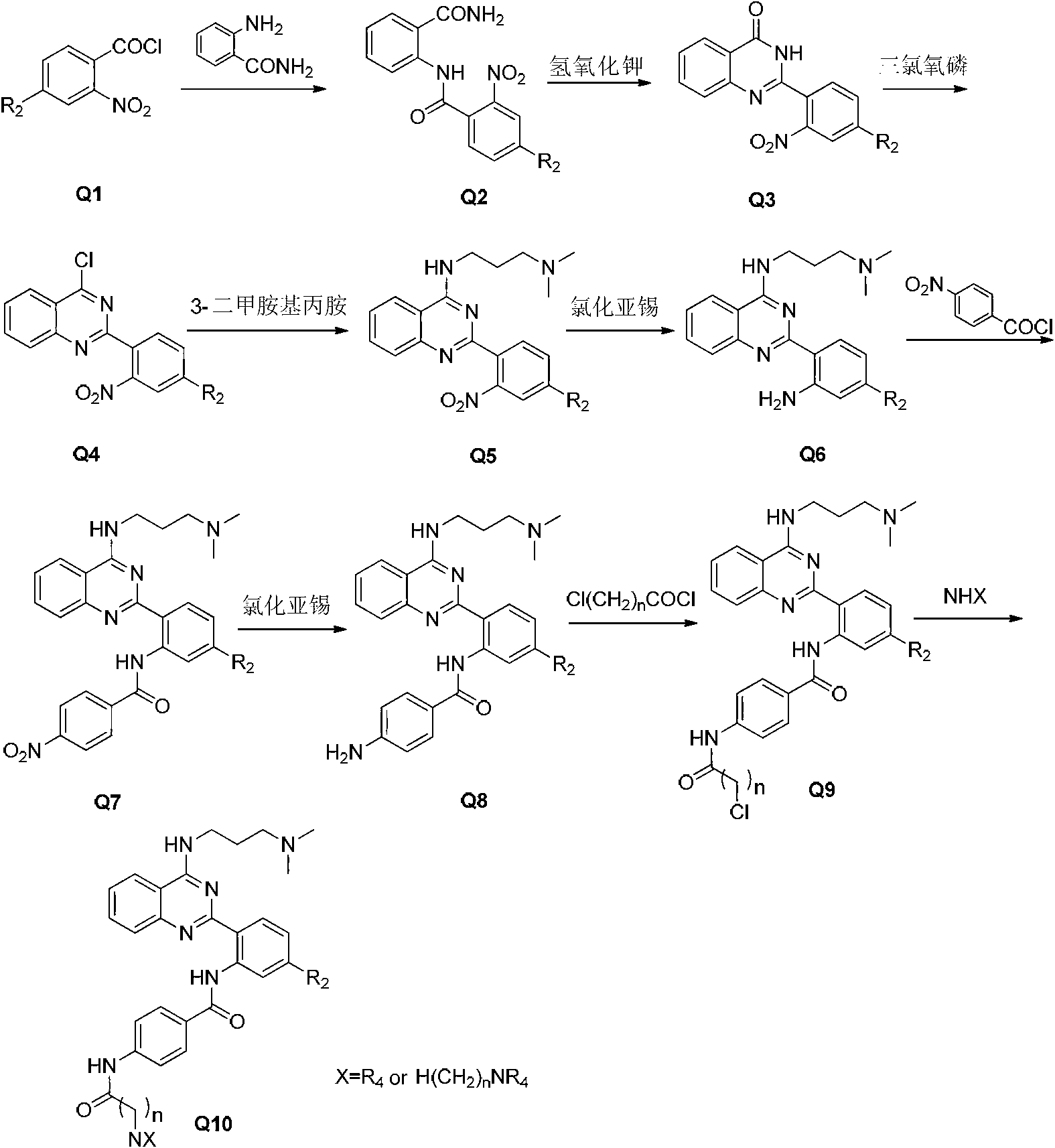
2-phenyl quinazoline derivative, preparation method thereof, and application in preparation of anti-cancer drugs
A technology of phenylquinazoline and derivatives, which is applied in the directions of antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problem that the binding stability of G-quadruplex needs to be improved, and achieve good binding ability and selection ability, Significant inhibitory effect, the effect of broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]Example 1: Synthesis of compound QH-2
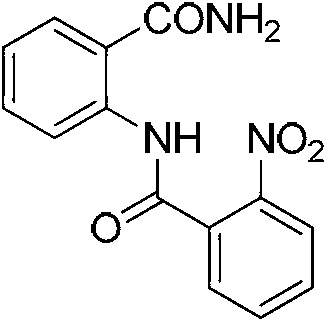
[0036] 100mmol of 2-nitrobenzoic acid was dissolved in 50ml of thionyl chloride, refluxed for 1.5h, and then the thionyl chloride was evaporated, and the obtained brown liquid was slowly added dropwise to 125mmol of anthranilamide and 250 mmol triethylamine in chloroform (250 ml) solution, stirred at room temperature for 6 h, filtered, washed with ethanol, and recrystallized with ethanol to obtain a white solid QH-2.
[0037] Yield: 88%; 1 H NMR(400MHz, DMSO)δ12.57(s,1H),8.53(d,J=8.2Hz,1H),8.43(s,1H),8.13(d,J=8.3Hz,1H),7.95–7.78 (m,5H),7.62(dd,J=11.4,4.0Hz,1H),7.29-7.22(m,1H).LC-MS m / z:286[M+H] + .
[0038]
[0039] Compound QH-2
Embodiment 2
[0040] Example 2: Synthesis of compound QC-2
[0041] The method is the same as in Example 1, except that 4-chloro-2-nitrobenzoic acid is used instead of 2-nitrobenzoic acid to obtain white solid QC-2.
[0042] Yield: 80%; 1 H NMR (400MHz, DMSO-d 6 )δ12.56(s,1H),8.44(d,J=8.2Hz,1H),8.39(s,1H),8.26(d,J=1.6Hz,1H),8.04–7.96(m,1H), 7.93–7.85(m, 2H), 7.81(s, 1H), 7.59(t, J=7.8Hz, 1H), 7.24(t, J=7.6Hz, 1H). LC-MS m / z: 320[M +H] + .
[0043]
[0044] Compound QC-2
Embodiment 3
[0045] Example 3: Synthesis of compound QH-3
[0046] After mixing 88 mmol of dry QH-2 with 100 ml of 10% potassium hydroxide aqueous solution and 100 ml of ethanol, react at 95°C for 3 to 4 hours. After the reaction was completed, ethanol was distilled off, and the pH value of the solution was adjusted to between 1 and 3 with hydrochloric acid, a large amount of white solids were precipitated, filtered and dried, and petroleum ether / ethyl acetate (volume ratio 3 / 1) was used as the eluent to pass through. Purification by silica gel chromatography gave QH-3 as a white solid.
[0047] Yield: 98%; 1 H NMR(400MHz, DMSO)δ12.86(s,1H),8.27-8.16(m,2H),7.95-7.81(m,4H),7.67(d,J=7.8Hz,1H),7.62-7.56( m,1H).LC-MS m / z:268[M+H] + .
[0048]
[0049] Compound QH-3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com