Process for synthesizing triazole derivative
A technology of triazole derivatives and synthesis process, applied in the direction of organic chemistry, etc., can solve the problems of harsh reaction conditions, long production cycle, complicated process, etc., and achieve the effects of mild reaction conditions and short time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
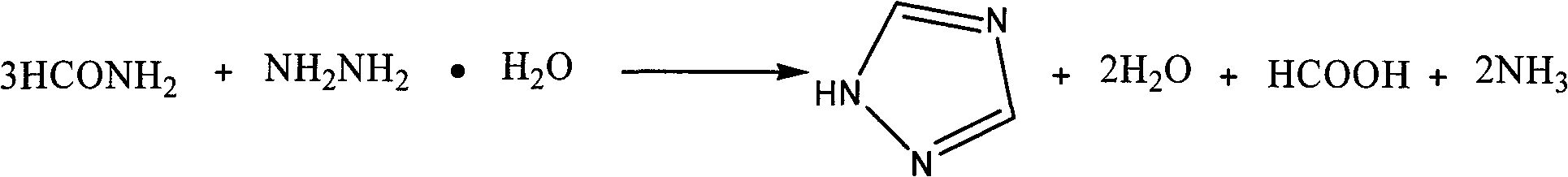
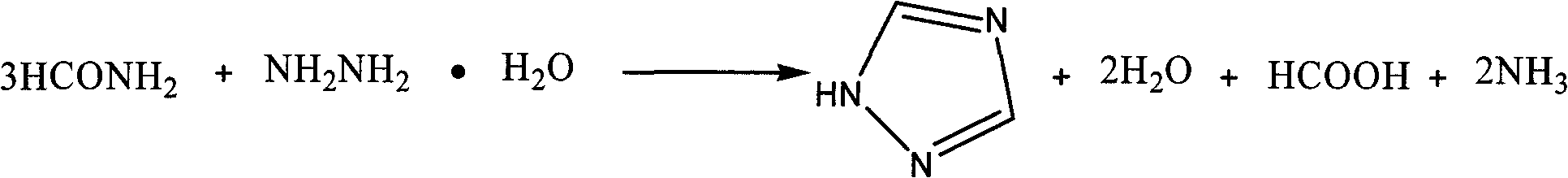
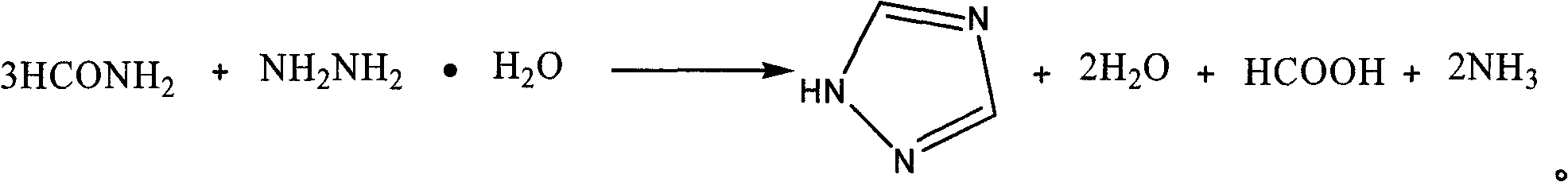
[0016] The process steps of the present invention are: adding formamide into a four-necked flask equipped with a stirrer, a constant pressure dropping funnel, a distillation device and an exhaust gas absorption device, heating to 175-190°C, adding dropwise hydrazine hydrate, 1 / 3 formamide The molar ratio of amide to hydrazine hydrate is 1:1.1 to 1:2, after 0.5 to 2.5 hours of dripping, introduce the escaped ammonia gas into the absorption bottle (containing 20%-30%H 2 SO 4 ) absorption; after the dropwise addition, keep warm for 0.5-2.5h; cool, and after a large amount of crystals are precipitated, filter with suction to obtain a crude product, wash with ethyl acetate to obtain the target product 1H-1,2,4-triazole, and calculate the product rate, measuring melting point.
[0017] The reaction equation is:
[0018]
[0019] The present invention selects the mol ratio of 1 / 3 formamide and hydrazine hydrate, reaction temperature, dripping time, soaking time as 1H-1,2, the in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com