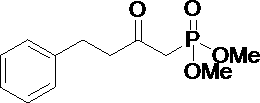
Method for preparing bimatoprost midbody
A technology of mixture and tetrahydrofuran, which is applied in the field of preparation of bimatoprost intermediates, can solve the problems of difficult industrial scale-up production and harsh reaction conditions, and achieve the effects of easy post-processing, simple operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A method for preparing 2-oxo-4-phenylbutyl dimethyl phosphate, comprising the steps of:
[0030] The first step, prepare lithium diisopropylamide tetrahydrofuran solution
[0031] (1) Under stirring, add 14.6 g of diisopropylamine and 30 mL of anhydrous tetrahydrofuran into a 250 mL reactor and mix uniformly to prepare mixture a;
[0032] (2) Under stirring, gradually add (2.5 M, 98.87 mL) n-butyllithium n-hexane solution dropwise to mixture a in an ice-water bath. After the dropwise addition, control the temperature at 0°C and continue stirring, and stop the reaction after 30 min. Stirring makes diisopropylamide lithium tetrahydrofuran solution;
[0033] The second step, preparation of dimethyl 2-oxo-4-phenylbutyl phosphate
[0034] (1) Under stirring, add 0.112 mol of ethyl 3-phenylpropionate, 200 mL of anhydrous tetrahydrofuran, and 0.118 mol of dimethyl methyl phosphate into a 500 mL single-necked flask and mix uniformly to obtain mixture b;
[0035] (2) Un...
Embodiment 2
[0040] A method for preparing 2-oxo-4-phenylbutyl dimethyl phosphate, comprising the steps of:
[0041] The first step, prepare lithium diisopropylamide tetrahydrofuran solution
[0042] (1) Under stirring, add 292 g of diisopropylamine and 5 L of anhydrous tetrahydrofuran into a 10 L reactor and mix uniformly to prepare mixture a;
[0043] (2) Under stirring, gradually add (2.5 M, 2 L) n-butyllithium cyclohexane solution dropwise to mixture a in an ice-water bath. After the dropwise addition, control the temperature at 0°C and continue stirring for 100 min. Stop stirring to obtain diisopropylamide lithium tetrahydrofuran solution;
[0044] The second step, preparation of dimethyl 2-oxo-4-phenylbutyl phosphate
[0045] (1) Under stirring, add 2.24 mol of ethyl 3-phenylpropionate, 4L of anhydrous tetrahydrofuran, and 2.42 mol of dimethyl methyl phosphate into a 20L reactor and mix uniformly to prepare mixture b;
[0046] (2) Under stirring, gradually add the diisopropy...
Embodiment 3
[0051] A method for preparing 2-oxo-4-phenylbutyl dimethyl phosphate, comprising the steps of:
[0052] The first step, prepare lithium diisopropylamide tetrahydrofuran solution
[0053] (1) Under stirring, add 58.4 g of diisopropylamine and 120 mL of anhydrous tetrahydrofuran into a 500 mL reactor and mix uniformly to prepare mixture a;
[0054] (2) Under stirring, gradually add (2.5 M, 395.5 mL) n-butyllithium n-hexane solution dropwise to mixture a under an ice-water bath. After the dropwise addition, control the temperature at 0°C and continue stirring for 60 min. Stop stirring to obtain diisopropylamide lithium tetrahydrofuran solution;
[0055] The second step, preparation of dimethyl 2-oxo-4-phenylbutyl phosphate
[0056] (1) Under stirring, add 0.448 mol of ethyl 3-phenylpropionate, 800 mL of anhydrous tetrahydrofuran, and 0.472 mol of dimethyl methyl phosphate into a 3L single-necked flask and mix uniformly to obtain mixture b;
[0057] (2) Under stirring, gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com