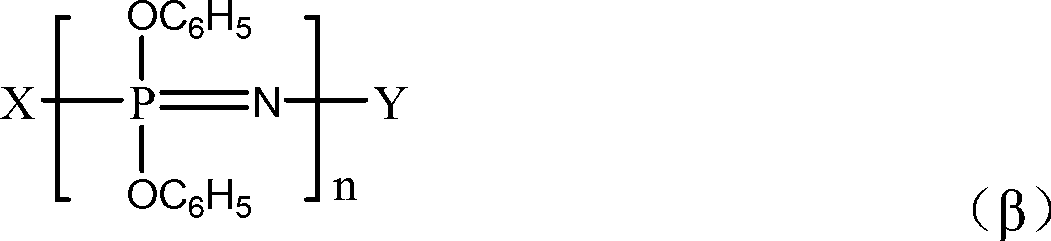Halogen-free flame retardant resin composition and use thereof
A technology of resin composition and flame retardant resin, which can be applied to other household appliances, synthetic resin layered products, applications, etc., can solve the problem that the performance of resin cured products is difficult to further improve, and achieve low amine value, low activity, and high performance. Effect of Tg and heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0099] A halogen-free flame-retardant resin composition, comprising:
[0100] (A) Phenoxyphosphazene compound (A 1 ) with compounds having a dihydrobenzoxazine ring (A 2 ) mixture: 45 parts by weight, phenoxyphosphazene compound (A 1 ) with compounds having a dihydrobenzoxazine ring (A 2 ) with a weight ratio of 1:25; (B) bisphenol A type epoxy resin with an epoxy equivalent of 1500: 45 parts by weight; (C) nitrogen-containing phenolic resin: 10 parts by weight, and (D) diaminodiphenyl Ether: 0.5 parts by weight.
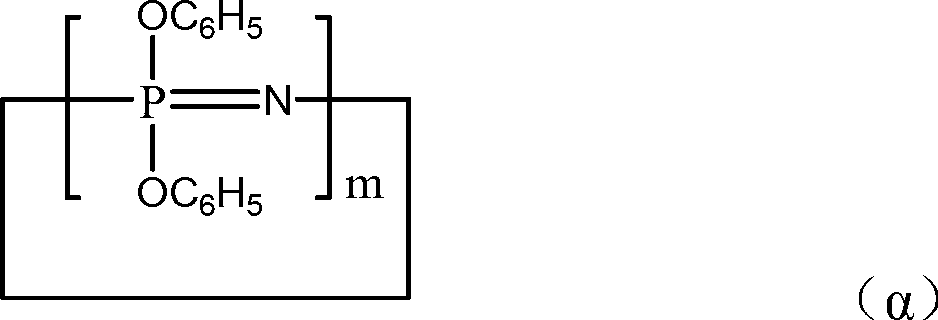
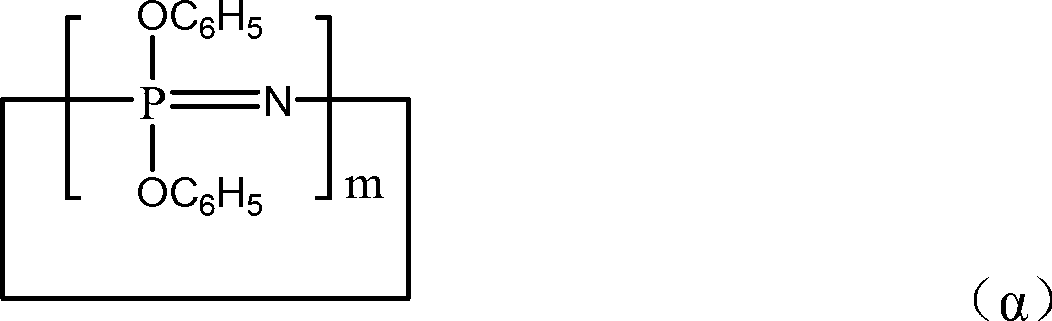
[0101] A 1 The softening point (100~110°C) is a mixture of cyclic phenoxyphosphazene compounds and chain phenoxyphosphazene compounds shown in the following structural formula:
[0102]
[0103] m is an integer from 3 to 25; X is -N=P(OC 6 h 5 ) 3 ; Y is -P(OC 6 h 5 ) 4 ; n is an integer of 3-100.
[0104] A 2 Be the bisphenol A type benzoxazine resin described in following structural formula:
[0105]
[0106] R is -C(CH 3 ) 2 -, R 1 for
Embodiment 14
[0108] A halogen-free flame-retardant resin composition, comprising:
[0109] (A) Phenoxyphosphazene compound (A 1 ) with compounds having a dihydrobenzoxazine ring (A 2 ) mixture: 90 parts by weight, phenoxyphosphazene compound (A 1 ) with compounds having a dihydrobenzoxazine ring (A 2 ) in a weight ratio of 1:2; (B) bisphenol A type epoxy resin with an epoxy equivalent of 500: 30 parts by weight; (C) phenol novolac resin: 20 parts by weight, and (D) diaminodiphenyl sulfone : 10 parts by weight.
[0110] A 1 The softening point (100~110°C) is a mixture of cyclic phenoxyphosphazene compounds and chain phenoxyphosphazene compounds shown in the following structural formula:
[0111]
[0112] m is an integer from 3 to 25; X is -N=P(O)C 6 h 5 ; Y is -P(O)(C 6 h 5 ) 2 ; n is an integer of 3-100.
[0113] A 2 Be the bisphenol F type benzoxazine resin described in following structural formula:
[0114]
Embodiment 15
[0116] A halogen-free flame-retardant resin composition, comprising:
[0117] (A) Phenoxyphosphazene compound (A 1 ) with compounds having a dihydrobenzoxazine ring (A 2 ) mixture: 65 parts by weight, phenoxyphosphazene compound (A 1 ) with compounds having a dihydrobenzoxazine ring (A 2 ) in a weight ratio of 1:10; (B) bisphenol A type epoxy resin with an epoxy equivalent of 1000: 10 parts by weight; (C) bisphenol A type phenolic resin: 25 parts by weight, and (D) m-benzene Dimethylamine: 5 parts by weight.
[0118] A 1 The softening point (100~110°C) is a mixture of cyclic phenoxyphosphazene compounds and chain phenoxyphosphazene compounds shown in the following structural formula:
[0119]
[0120] m is an integer from 3 to 25; X is -N=P(OC 6 h 5 ) 3 ; Y is -P(OC 6 h 5 ) 4 ; n is an integer of 3-100.
[0121] A 2 It is a phenolphthalein type benzoxazine resin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com