Copper ion fluorescence probe and synthetic method thereof
A fluorescent probe, copper ion technology, applied in the field of analytical chemistry, can solve the problems of large fluorescence influence, cytotoxicity, difficult to accurately measure metal ions, etc., and achieve the effect of good application prospect and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
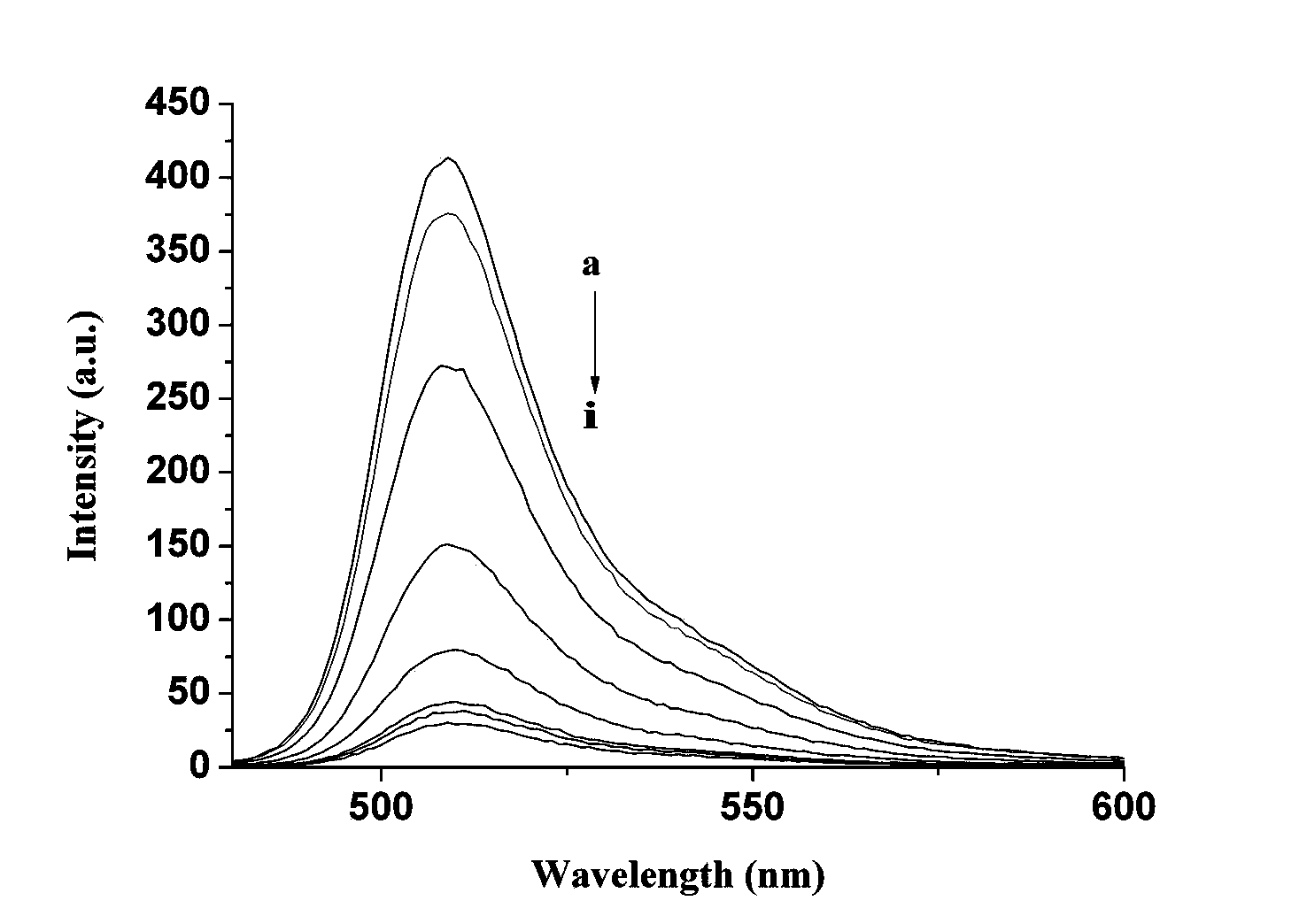
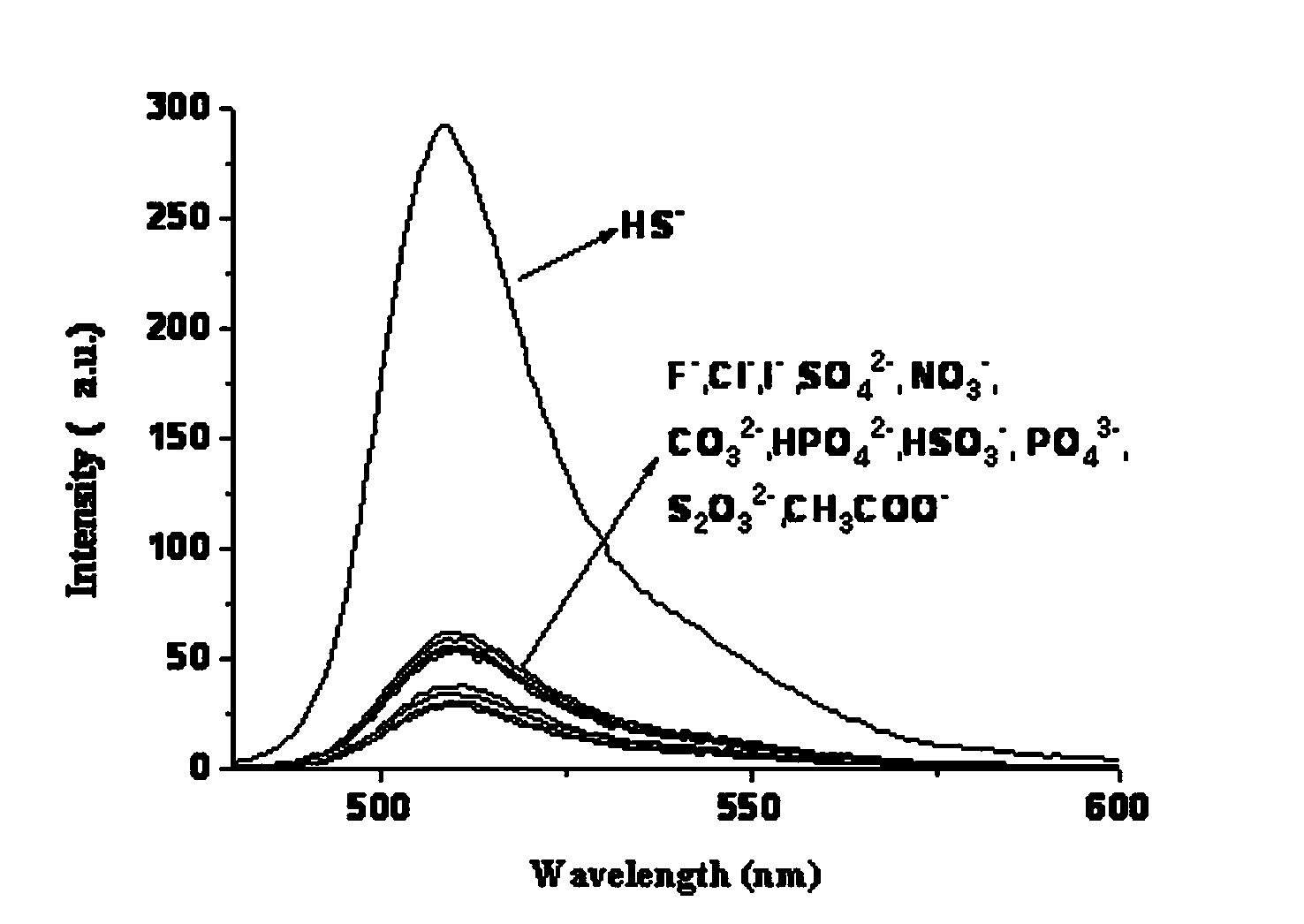
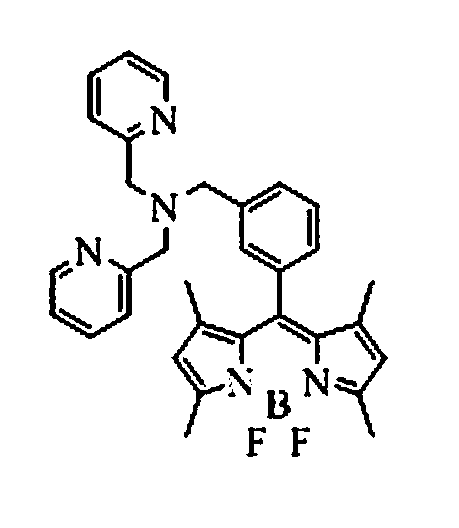
[0031] Add CH 2 Cl 2 100ml, react at 40°C for 4h; then add boron trifluoride (BF 3 ) 0.58ml (0.46mmol), react at 40°C for 6h; then add bis(2-pyridylmethyl)amine (83mg, 0.45mmol), triethylamine (378mg, 0.45mmol), nitrogen protection, 80°C oil bath reflux for 8h ; After the reaction, use 3 × 30ml saturated sodium chloride solution to extract and wash, use 3 × 30ml dichloromethane to extract the aqueous phase, combine the organic phases, dry over anhydrous sodium sulfate, and rotary evaporate the solvent to obtain a brown oil; Silica gel column Chromatographic separation, eluting with ethyl acetate, gave dark red viscous solid 8-[bis(2-pyridylmethyl)amine-3-benzyl]-4,4-difluoro-1,3,5, 7-tetramethyl-4-boron-3a,4a-dipyrrole (BODIPY-DPA) 75mg, yield 73%. Molecular formula C 32 h 32 BF 2 N 5 Elemental analysis measured value C, 71.81; H, 6.03; N, 13.06, theoretical value C, 71.78; H, 6.02; N, 13.08%; 1 HNMR (400MHz, CDCl 3 )δ H 8.54(2H,d),7.68(2H,t),7.54(3H,d),7.45(2H,d),7....
Embodiment 2
[0033] Add CH 2 Cl 2 200ml, react at 40°C for 8h; then add boron trifluoride (BF 3 ) 0.58ml (0.46mmol), react at 40°C for 6h; then add bis(2-pyridylmethyl)amine (83mg, 0.45mmol), triethylamine (378mg, 0.45mmol), nitrogen protection, 80°C oil bath reflux for 8h ; After the reaction, use 3 × 30ml saturated sodium chloride solution to extract and wash, use 3 × 30ml dichloromethane to extract the aqueous phase, combine the organic phases, dry over anhydrous sodium sulfate, and rotary evaporate the solvent to obtain a brown oil; Silica gel column Chromatographic separation, eluting with ethyl acetate, gave dark red viscous solid 8-[bis(2-pyridylmethyl)amine-3-benzyl]-4,4-difluoro-1,3,5, 7-Tetramethyl-4-boron-3a,4a-dipyrrole (BODIPY-DPA) 21mg, yield 22%.
Embodiment 3
[0035] Add CH 2 Cl 2 100ml, react at 40°C for 4h; then add boron trifluoride (BF 3 ) 0.58ml (0.46mmol), react at 40°C for 6h; then add bis(2-pyridylmethyl)amine (83mg, 0.45mmol), triethylamine (378mg, 0.45mmol), nitrogen protection, 80°C oil bath reflux for 8h ; After the reaction, use 3 × 30ml saturated sodium chloride solution to extract and wash, use 3 × 30ml dichloromethane to extract the aqueous phase, combine the organic phases, dry over anhydrous sodium sulfate, and rotary evaporate the solvent to obtain a brown oil; Silica gel column Chromatographic separation, eluting with ethyl acetate, gave dark red viscous solid 8-[bis(2-pyridylmethyl)amine-3-benzyl]-4,4-difluoro-1,3,5, 7-Tetramethyl-4-boron-3a,4a-dipyrrole (BODIPY-DPA) 34mg, yield 33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com