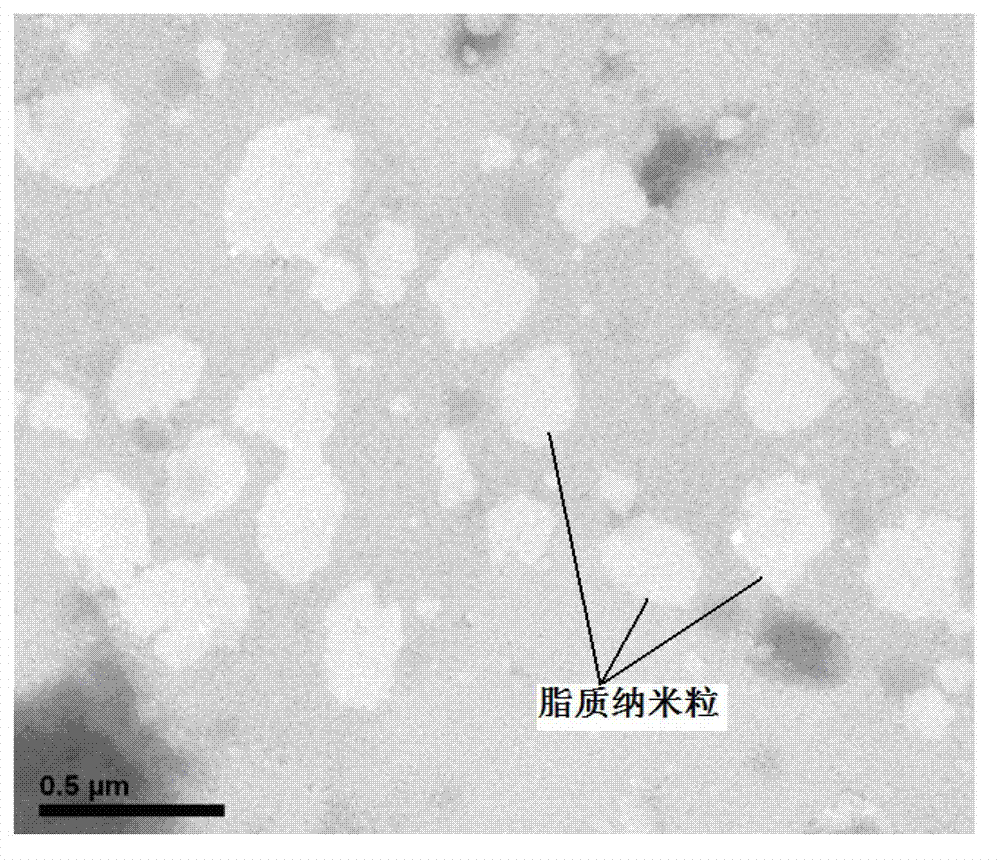
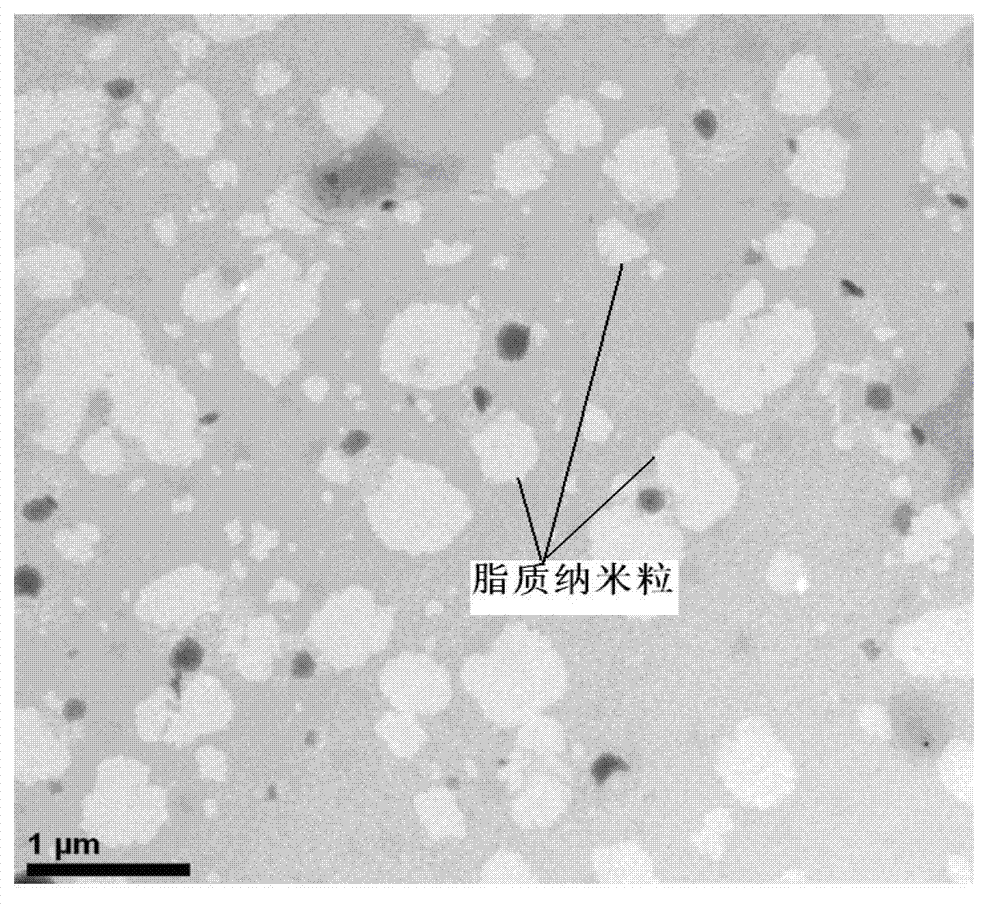
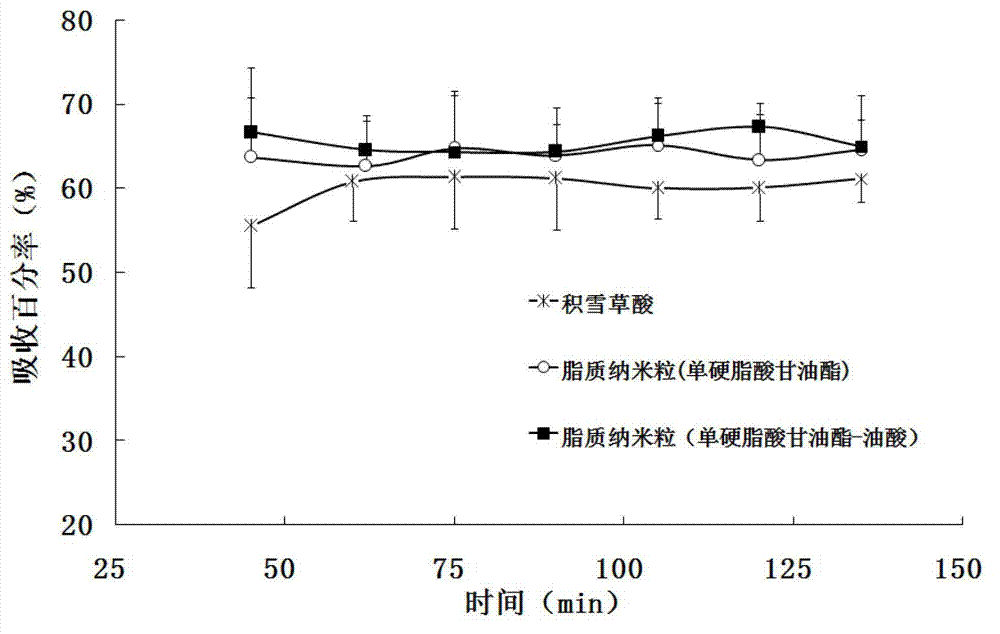
Asiatic acid lipid nanoparticle capable of stimulating oral absorption and preparation method thereof
A technology of lipid nanoparticles and asiatic acid, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., to improve oral bioavailability, simple preparation process, and simple prescription composition Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The above-mentioned preparation method of asiatic acid lipid nanoparticles comprises the following process:
[0020] 1. Using absolute ethanol as a solvent, add lipid materials and asiatic acid (chromatographic purity greater than 90%), heat and dissolve in a water bath at (70±2) ° C, as an organic phase, in which the mass of asiatic acid and lipid materials The ratio is 1:7-11, and the solution concentration of the lipid material is 7-11 mg / mL.
[0021] 2. With water as the dispersed phase, heat to (70±2)°C.
[0022] 3. Under 400r / min mechanical stirring, inject the organic phase into the dispersed phase, the volume ratio of absolute ethanol to water is 1:10; continue stirring for 5 minutes to obtain a cloudy liquid with opalescence. After the turbid solution was cooled to room temperature, the pH was adjusted to 1.2 with hydrochloric acid, centrifuged, the precipitate was washed with a small amount of deionized water, centrifuged again, and the upper liquid was disca...
Embodiment 1
[0024] Example 1: Preparation of asiatic acid glyceryl monostearate lipid nanoparticles (mass ratio of main drug to lipid material is 1:7)
[0025] Accurately weigh 5 mg of asiatic acid and 35 mg of glyceryl monostearate (commercially available, purity > 90%), dissolve in 5 mL of absolute ethanol under heating in a water bath at (70±2) °C, and use it as the organic phase, lipid material The concentration of the solution is 7mg / mL. With water as the dispersed phase, heat to (70±2)°C. Under mechanical stirring at 400 rpm, inject the organic phase into 50 mL of the dispersed phase, and continue stirring for 5 min to obtain a cloudy liquid with opalescence. After the turbid solution was allowed to cool to room temperature, the pH was adjusted to 1.2 with hydrochloric acid, centrifuged, the precipitate was washed with a small amount of deionized water, and the supernatant liquid was discarded by centrifugation to obtain asiatic acid lipid nanoparticles. The nanoparticles can be d...
Embodiment 2
[0029] Example 2: Preparation of asiatic acid glyceryl monostearate lipid nanoparticles (mass ratio of main drug to lipid material is 1:9)
[0030] Accurately weigh 5 mg of asiatic acid and 45 mg of glyceryl monostearate (commercially available, purity> 90%), (70
[0031] ±2) Dissolve in 5mL of absolute ethanol under heating in a water bath at ℃, and use as the organic phase, the solution concentration of the lipid material is 9mg / mL. Use water as the dispersed phase, heat to (70±2)°C, and under mechanical stirring at 400rpm, inject the organic phase into 50mL of the dispersed phase, and continue stirring for 5min to obtain a cloudy liquid with opalescence. After the turbid solution was cooled to room temperature, the pH was adjusted to 1.2 with hydrochloric acid, centrifuged, the precipitate was washed with a small amount of deionized water, centrifuged again, and the upper liquid was discarded to obtain asiatic acid lipid nanoparticles. The nanoparticles can be dispersed in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com