Asiatic acid lipid nanoparticle capable of stimulating oral absorption and preparation method thereof
A technology of lipid nanoparticles and asiatic acid, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., to achieve simple composition of prescriptions, good process reproducibility, and preparation process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
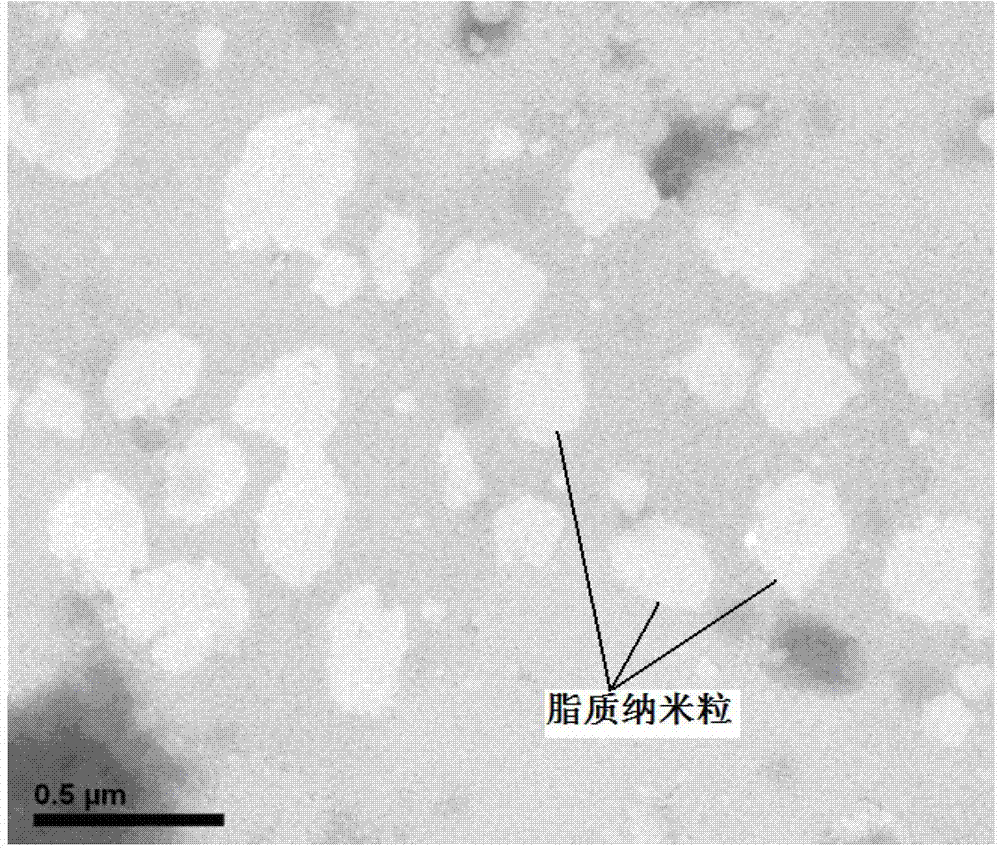
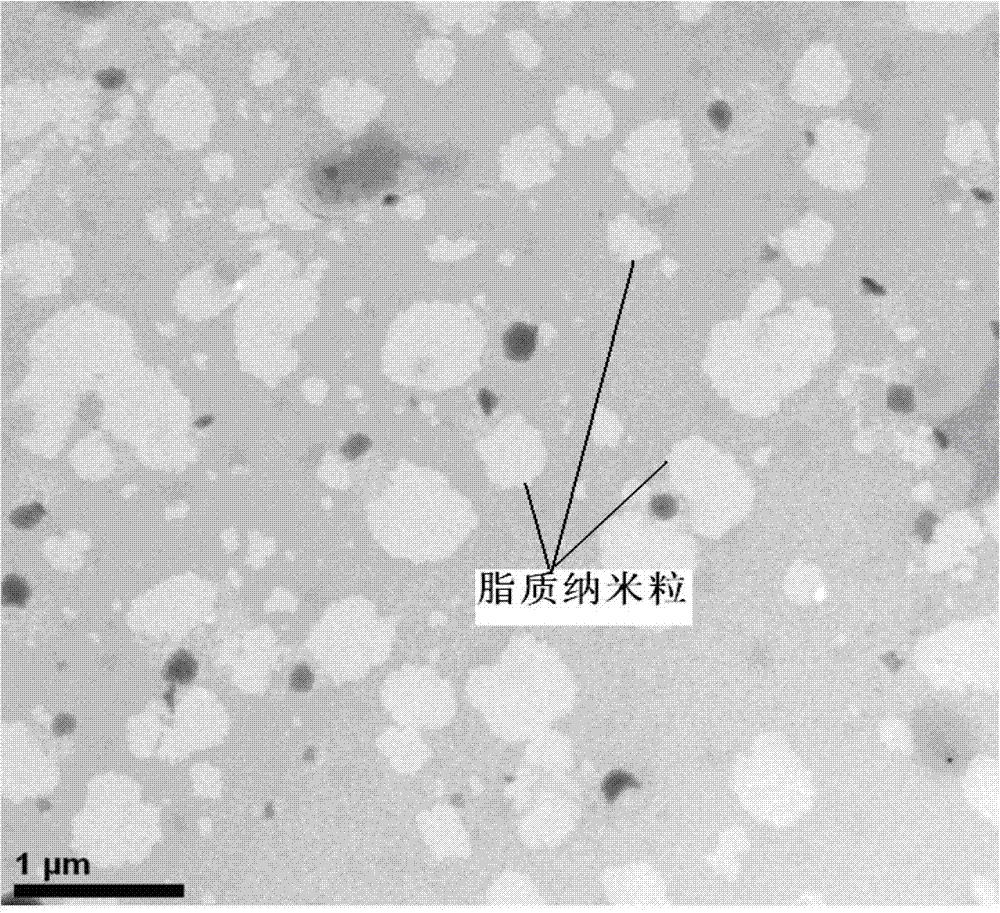
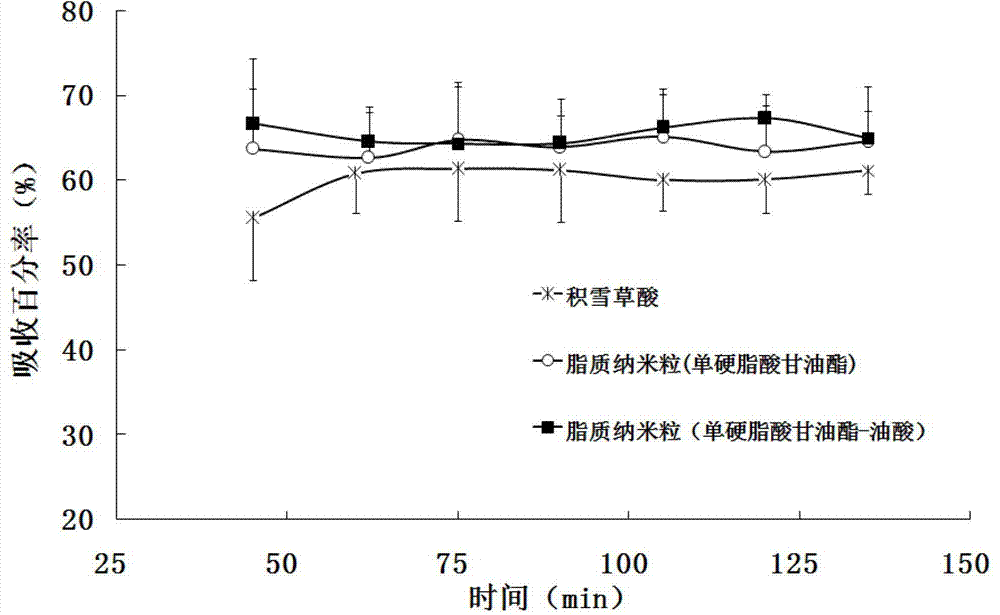
Image
Examples
preparation example Construction
[0019] The above-mentioned preparation method of asiatic acid lipid nanoparticles comprises the following process:
[0020] 1. Using absolute ethanol as a solvent, add lipid materials and asiatic acid (chromatographic purity greater than 90%), heat and dissolve in a water bath at (70±2) ° C, as an organic phase, in which the mass of asiatic acid and lipid materials The ratio is 1:7-11, and the solution concentration of the lipid material is 7-11 mg / mL.
[0021] 2. With water as the dispersed phase, heat to (70±2)°C.
[0022] 3. Under 400r / min mechanical stirring, inject the organic phase into the dispersed phase, the volume ratio of absolute ethanol to water is 1:10; continue stirring for 5 minutes to obtain a cloudy liquid with opalescence. After the turbid solution was cooled to room temperature, the pH was adjusted to 1.2 with hydrochloric acid, centrifuged, the precipitate was washed with a small amount of deionized water, centrifuged again, and the upper liquid was disca...
Embodiment 1
[0024] Example 1: Preparation of asiatic acid glyceryl monostearate lipid nanoparticles (mass ratio of main drug to lipid material is 1:7)
[0025] Accurately weigh 5 mg of asiatic acid and 35 mg of glyceryl monostearate (commercially available, purity > 90%), dissolve in 5 mL of absolute ethanol under heating in a water bath at (70±2) °C, and use it as the organic phase, lipid material The concentration of the solution is 7mg / mL. With water as the dispersed phase, heat to (70±2)°C. Under mechanical stirring at 400 rpm, inject the organic phase into 50 mL of the dispersed phase, and continue stirring for 5 min to obtain a cloudy liquid with opalescence. After the turbid solution was allowed to cool to room temperature, the pH was adjusted to 1.2 with hydrochloric acid, centrifuged, the precipitate was washed with a small amount of deionized water, and the supernatant liquid was discarded by centrifugation to obtain asiatic acid lipid nanoparticles. The nanoparticles can be d...
Embodiment 2
[0029] Example 2: Preparation of asiatic acid glyceryl monostearate lipid nanoparticles (mass ratio of main drug to lipid material is 1:9)
[0030] Accurately weigh 5 mg of asiatic acid and 45 mg of glyceryl monostearate (commercially available, purity> 90%), (70
[0031] ±2) Dissolve in 5mL of absolute ethanol under heating in a water bath at ℃, and use as the organic phase, the solution concentration of the lipid material is 9mg / mL. Use water as the dispersed phase, heat to (70±2)°C, and under mechanical stirring at 400rpm, inject the organic phase into 50mL of the dispersed phase, and continue stirring for 5min to obtain a cloudy liquid with opalescence. After the turbid solution was cooled to room temperature, the pH was adjusted to 1.2 with hydrochloric acid, centrifuged, the precipitate was washed with a small amount of deionized water, centrifuged again, and the upper liquid was discarded to obtain asiatic acid lipid nanoparticles. The nanoparticles can be dispersed in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com