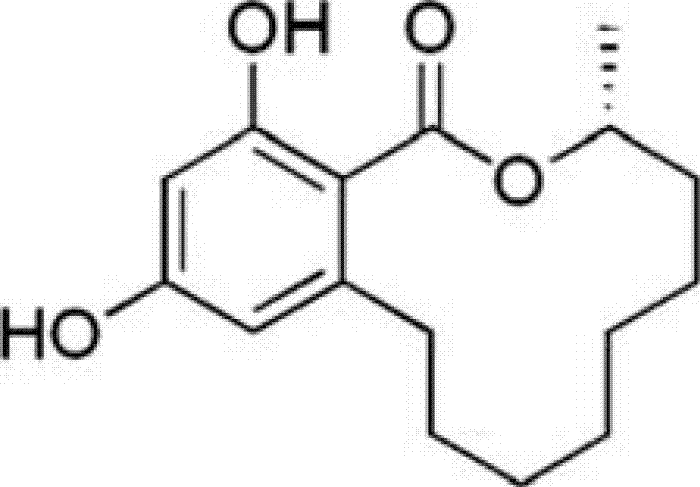
Application of des-O-methyllasiodiplodin in preparation of medicament for treating kidney diseases
A technology of methylmadiprene and kidney disease, which is applied in the application field of de-O-methylmadiprene in the preparation of medicines for treating kidney disease, and can solve the problems of no research reports on the therapeutic effect of kidney disease , to achieve a good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
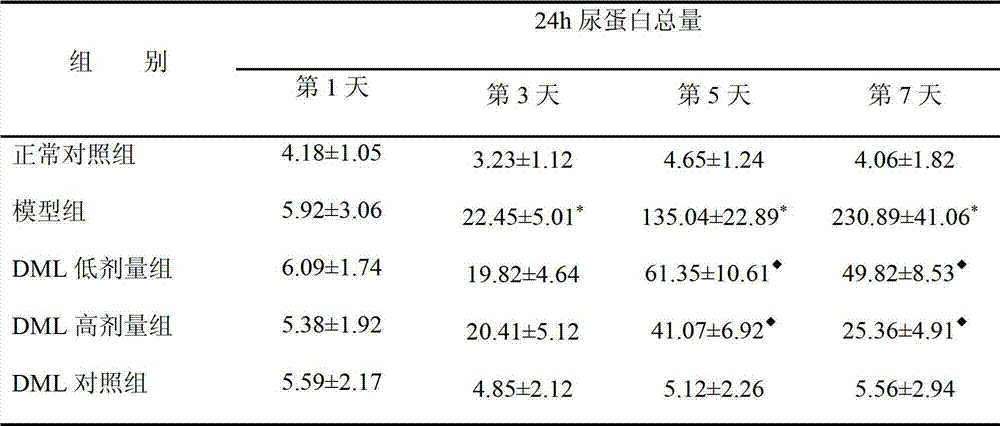
[0019] Embodiment 1: The pharmacological effect of des-O-methylmadiprene on nephrotic syndrome
[0020] 1. Experimental materials
[0021] Aminonucleoside puromycin was purchased from Sigma Company of the United States; SuperScript III RNase H-Reverse Transcriptase Kit was purchased from Invitrogen Company of the United States; goat anti-nephrin, podocin antibody, horseradish peroxidase-labeled anti-goat and mouse secondary antibodies were purchased from American Santa Cruz company.
[0022] 2. Experimental method
[0023] 1. Animal model preparation and experimental grouping: 40 male SD rats, weighing 80-100 grams. Randomly divided into 5 groups, normal control group, model group, de-O-methylmaidepredrine (DML) low-dose treatment group, de-O-methylmaudepredrine (DML) high-dose treatment group and de-O-methylmaudepredrine (DML) control group, 8 rats in each group (n=8). Normal control group: intraperitoneal injection of normal saline; model group, des-O-methylmaideprene lo...
Embodiment 2
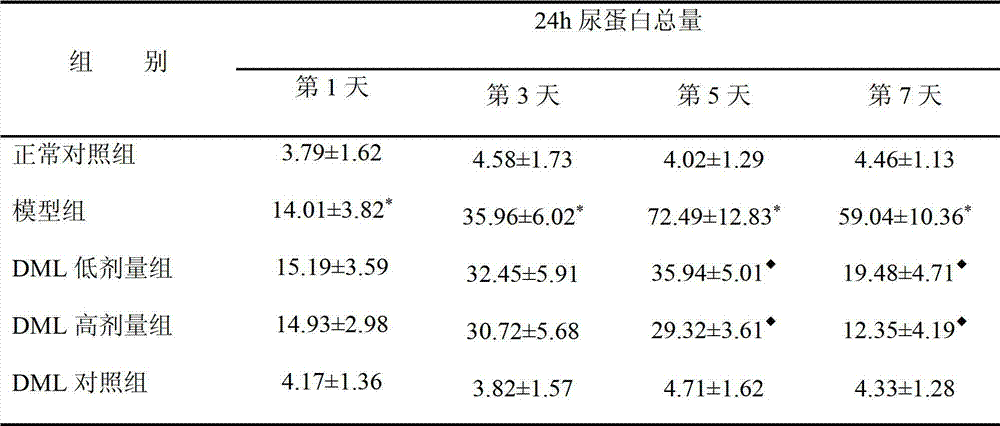
[0038] Example 2 Pharmacological effects of des-O-methylmaudeprene (DML) on mesangial proliferative glomerulonephritis.
[0039] 1. Experimental materials
[0040] 40 healthy male SD rats, 4 weeks old, weighing about 160g, were purchased from the Experimental Animal Center of Jiangsu Province; complete Freund's adjuvant, FITC-labeled goat anti-rabbit IgG and Schiff reagent were purchased from Sigma Company of the United States, proliferating cell nuclear antigen ( PCNA) and monocyte chemoattractant-1 (MCP-1) monoclonal antibody were purchased from Sant Cruz Company in the United States, horseradish peroxidase-labeled goat anti-rabbit IgG, normal goat serum blocking solution, DAB chromogenic reagent were purchased from Beijing Zhongshan Biotechnology Company.
[0041] 2. Experimental method
[0042] 1. Preparation of anti-rat thymocyte antiserum (ATS): 4-week-old SD rats, take their thymus and prepare a certain concentration of single thymocyte suspension, mix with complete F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com