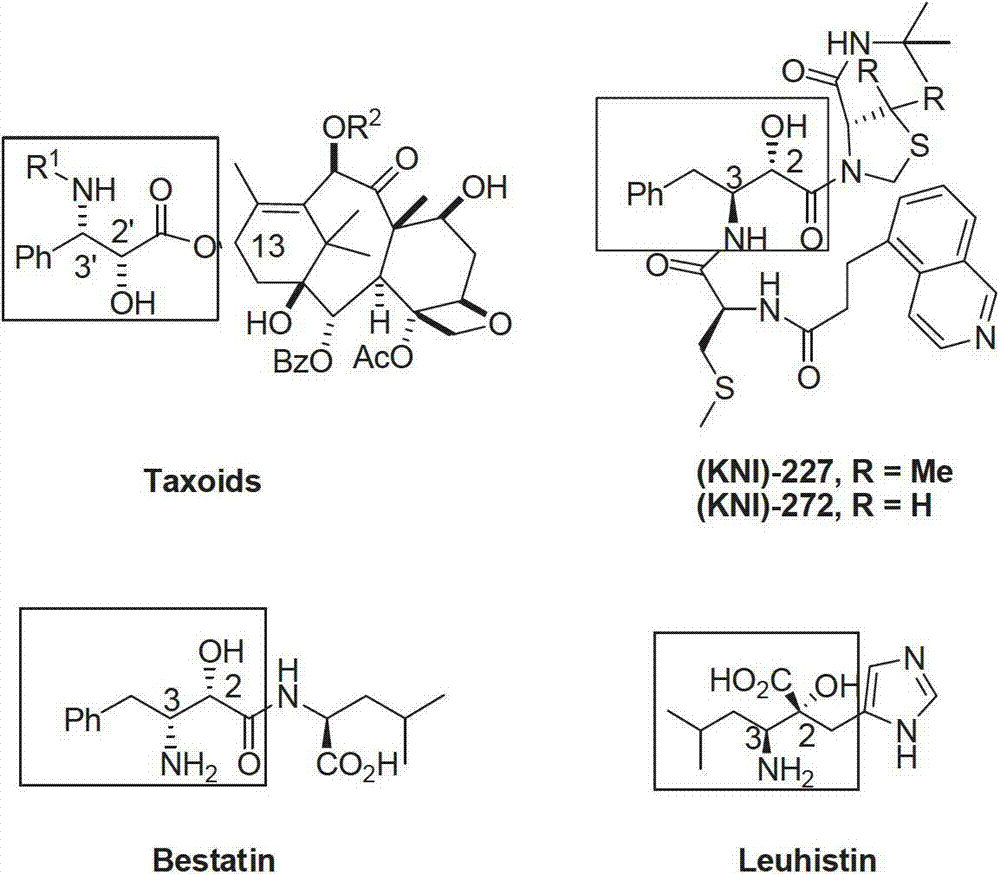
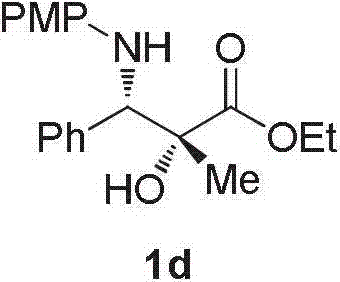
Preparation method of optical pure alpha-hydroxyl-beta-aminopropionic acid ester derivative
A technology of aminopropionate and derivatives, which is applied in the field of preparation of α-hydroxy-β-alanine ethyl derivatives, can solve the problems of unfavorable industrial production and high production costs, and achieve the improvement of technical cost and production efficiency, and easy The effect of stable operation and performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of (2S, 3S)-2-hydroxyl-3-anilinophenylpropionic acid ethyl ester
[0029] Weigh N-phenylbenzaldimine (1.0mmol), rhodium acetate (0.01mol), (R)-3,3'-(ditriphenylsilyl)-binaphthol phosphoric acid (0.02mol), Add it into a 20mL two-necked bottle, add commercially available reagent grade solvent dichloromethane, stir and configure a mixed solution with a total amount of 8mL, that is, make a mixed solution of imine, rhodium acetate, water and solvent. Measure water (2.0mmol) with a micro-injector, inject it into a two-neck bottle, stir well and mix evenly. Weigh ethyl diazoacetate (2.0mmol), add dichloromethane to make 4mL mixed solution (diazo solution), draw the mixed solution with a 5mL syringe, inject the diazo solution into the diazo solution for 1 hour by peristaltic pump control In the dichloromethane solution of amine, rhodium acetate and water, after stirring at room temperature for 1 hour, the dichloromethane was removed by rotary evaporation ...
Embodiment 2
[0031] Example 2 Preparation of (2S, 3S)-2-methyl-2-hydroxyl-3-anilinophenylpropionic acid isopropyl ester
[0032] Weigh N-phenylbenzaldimine (1.0mmol), rhodium acetate (0.01mol), (R)-3,3'-(ditriphenylsilyl)-binaphthol phosphoric acid (0.02mol), Add it to a 20mL two-necked bottle, add commercially available reagent grade dichloromethane, and stir to form a mixed solution with a total volume of 8mL. Measure water (2.0mmol) with a micro-injector, inject it into a two-neck bottle, stir well and mix evenly. Weigh isopropyl diazopropionate (2.0mmol), add dichloromethane to form a 4mL mixed solution, draw the mixed solution with a 5mL syringe, and inject imine, rhodium acetate and water dichloromethane via a peristaltic pump control for 1 hour. In the methane solution, after stirring at room temperature for 1 hour, dichloromethane was removed by rotary evaporation at 40°C, and then the pure target product 1b was isolated by column chromatography (eluent: petroleum ether: ethyl ace...
Embodiment 3
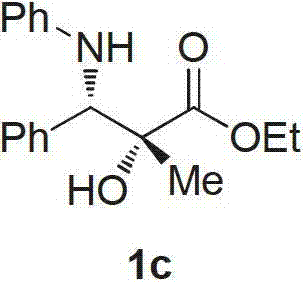
[0034] Example 3 Preparation of (2S, 3S)-2-methyl-2-hydroxyl-3-anilinophenylpropionic acid ethyl ester
[0035] Weigh N-phenylbenzaldimine (1.0mmol), dichlorobis(4-isopropyltoluene) staple (0.01mol) (R)-3,3'-(ditriphenylsilyl)- Add binaphthol phosphoric acid (0.02mol) into a 20mL two-necked bottle, add commercially available reagent grade dichloromethane, and stir to form a mixed solution with a total volume of 8mL. Measure water (2.0mmol) with a micro-injector, inject it into a two-neck bottle, stir well and mix evenly. Weigh ethyl diazopropionate (2.0mmol), add dichloromethane to make 4mL mixed solution, draw the mixed solution with a 5mL syringe, and inject the dichloromethane of imine, rhodium acetate and water by peristaltic pump control for 1 hour. In the solution, after stirring at room temperature for 1 hour, dichloromethane was removed by rotary evaporation at 40°C, and then the pure target product 1c was isolated by column chromatography (eluent: petroleum ether: et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com