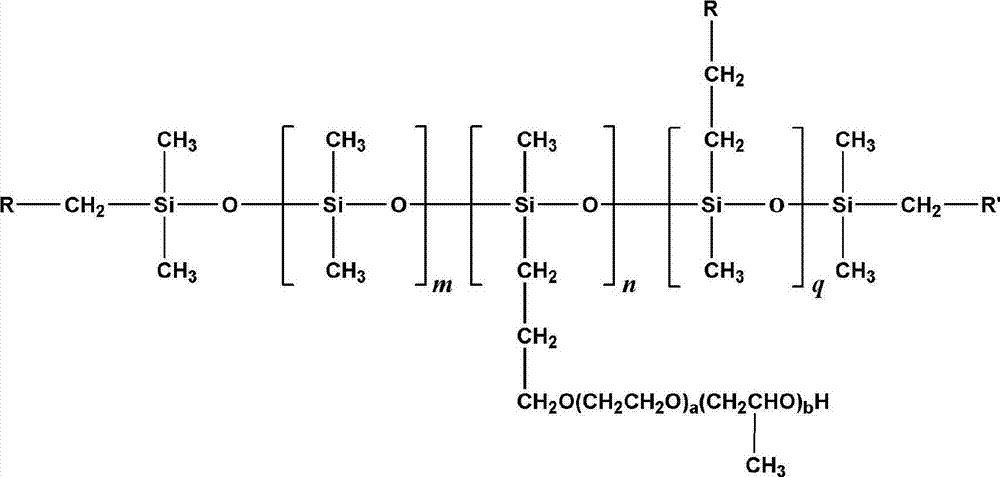
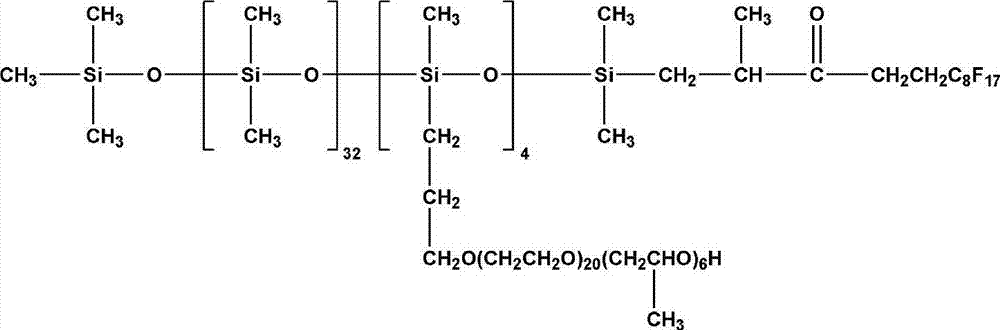
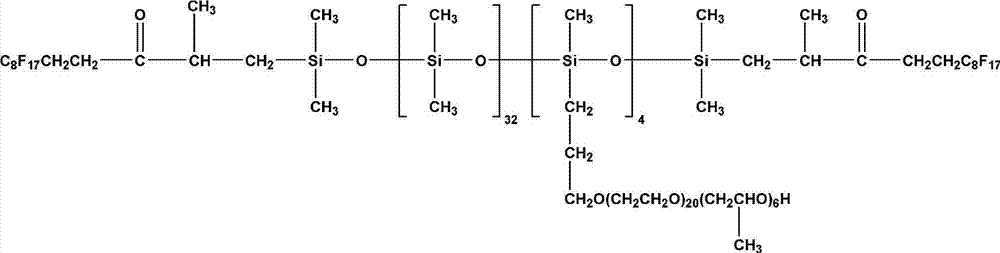
Fluorine contained organosilicon-polyether copolymer and preparation method thereof
A technology of polyether copolymer and silicone, which is applied in the field of silicone polyether copolymer and its preparation, can solve the problems of weakening effect, lower hydrophobic efficiency, and lower performance of fluorosilicon polymer, so as to reduce surface tension and reduce interference , the effect of good surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Add 5.00g of allyl alcohol, 76.00g of ethylene oxide and 30.00g of propylene oxide into the reactor, and react for 5h in the presence of 0.55g of KOH catalyst, pressure ≤ 0.4MPa, and reaction temperature of 120°C , to obtain terminal allyl polyether (I);
[0027] (2) React octamethylcyclotetrasiloxane, tetramethylcyclotetrasiloxane, tetramethyldihydrodisiloxane and hexamethyldisiloxane at 65°C for 5 hours under the action of sulfuric acid, Obtain single-end hydrogen-containing polymethylhydrogensiloxane (II), in which octamethylcyclotetrasiloxane, tetramethylcyclotetrasiloxane, tetramethyldihydrodisiloxane and hexamethyldisiloxane The molar ratio between oxanes is 8:1:0.5:0.5.
[0028] (3) 100.00g of single-end hydrogen-containing polymethylhydrogensiloxane (II) and 19.30g of heptadecanyl fluorodecyl methacrylate were added to the reactor, and 6ppm of chloroplatinic acid catalyst and 12ppm of N,N-dimethylaniline Co-catalyst, heated to 90-120°C under normal pressur...
Embodiment 2
[0033] (1) Add 5.00g of allyl alcohol, 76.00g of ethylene oxide and 30.00g of propylene oxide into the reactor, and react for 5h in the presence of 0.56g of KOH catalyst, pressure ≤ 0.4MPa, and reaction temperature of 120°C , to obtain terminal allyl polyether (I);
[0034](2) React octamethylcyclotetrasiloxane, tetramethylcyclotetrasiloxane and tetramethyldihydrodisiloxane at 65°C for 5 hours under the action of sulfuric acid to obtain hydrogen-containing polymethyl Hydrogen siloxane (II), wherein the molar ratio between octamethylcyclotetrasiloxane, tetramethylcyclotetrasiloxane and tetramethyldihydrodisiloxane is 8:1:1.
[0035] (3) 100.00g of hydrogen-containing polymethylhydrogensiloxane (II) at both ends and 38.80g of heptadecanyl fluorodecyl methacrylate were added to the reactor, and 7ppm of chloroplatinic acid catalyst and 14ppm of N,N-dimethylaniline Co-catalyst, heated to 90-120°C under normal pressure and reacted for 4 hours to obtain polymethylhydrogensiloxane (I...
Embodiment 3
[0040] (1) Add 5.00g of allyl alcohol, 95.00g of ethylene oxide and 25.00g of propylene oxide into the reactor, and react for 5h in the presence of 0.63g of KOH catalyst, pressure ≤ 0.4MPa, and reaction temperature of 120°C , to obtain terminal allyl polyether (I);
[0041] (2) React octamethylcyclotetrasiloxane, tetramethylcyclotetrasiloxane, tetramethyldihydrodisiloxane and hexamethyldisiloxane at 60°C for 5 hours under the action of sulfuric acid, Obtain single-end hydrogen-containing polymethylhydrogensiloxane (II), in which octamethylcyclotetrasiloxane, tetramethylcyclotetrasiloxane and tetramethyldihydrodisiloxane and hexamethyldisiloxane The molar ratio of oxane is 6:1:0.5:0.5.
[0042] (3) Add 100.00g of single-end hydrogen-containing polymethylhydrogensiloxane (II) and 25.23g of perfluorodecylethylene into the reactor, heat up under normal pressure with 6ppm chloroplatinic acid catalyst and 13ppm triethylamine cocatalyst React at 90-120°C for 4 hours to obtain singl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface tension | aaaaa | aaaaa |
| Surface tension | aaaaa | aaaaa |
| Surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com