Method for preparing silver iodide by using iodine-contained brine
A technology of silver iodide and brine, which is applied in the direction of silver halide, can solve the problems of high cost of silver iodide and underutilization of iodine resources in brine, and achieve the effects of less reagent consumption, easy control of the production process, and low requirements for production scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
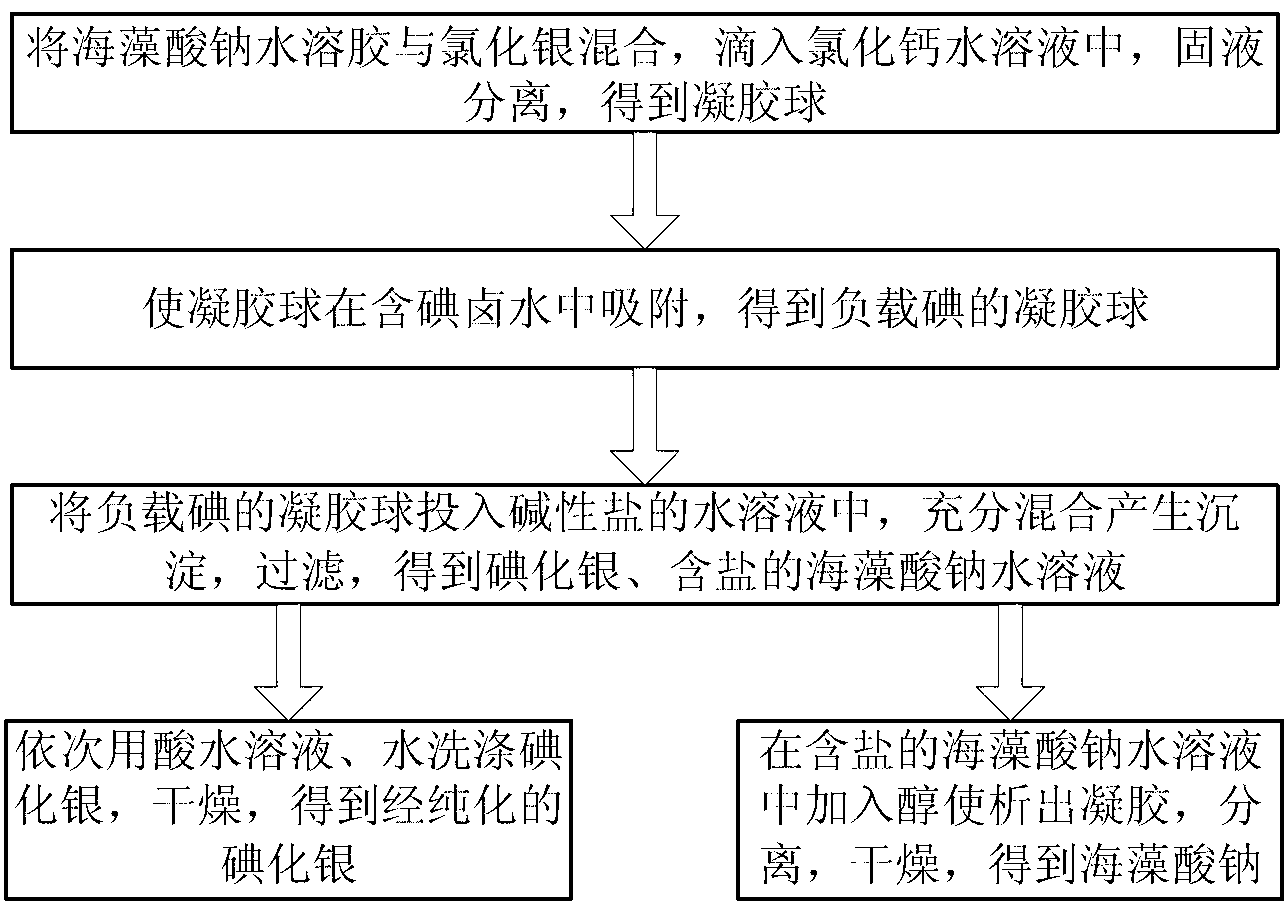
[0025] figure 1 According to an embodiment of the present invention, a process flow for preparing silver iodide using iodine-containing brine is shown. As can be seen from the figure, the preparation method mainly includes the following three steps:
[0026] Step A, preparing gel balls, mixing sodium alginate hydrosol and silver chloride at a mass ratio of 100-500:1 to form a sol mixture, dropping the sol mixture into an aqueous calcium chloride solution to produce a gel, The gel is separated from the liquid phase system to obtain gel balls, and the gel balls contain silver chloride and calcium alginate. The concentration of the sodium alginate hydrosol can be 0.5-8wt%, and the concentration of the calcium chloride aqueous solution can be 2-18wt%. If the mass ratio of sodium alginate hydrosol to silver chloride is too small, the silver chloride will fall off from the gel ball; if the mass ratio of sodium alginate hydrosol is too small, the adsorption capacity of the gel micr...
Embodiment 1
[0038] Preparation of gel balls: Mix 250g of 2wt% sodium alginate aqueous sol with 1g of silver chloride solid powder to form a sol mixture; drop the sol mixture into 5wt% calcium chloride aqueous solution to produce a gel precipitate; The gel precipitate was separated from the liquid phase system to obtain 7 g of gel spheres.
[0039]Adsorption of iodine ions: put the gel balls into 22 kg of brine containing 40 mg / L iodine, stir and adsorb for 48 hours to obtain 8 g of iodine-loaded gel balls.
[0040] Preparation of silver iodide: put 8g of iodine-loaded gel balls into 70g of 2wt% excess sodium carbonate aqueous solution, stir thoroughly for 30 minutes, and filter to obtain sodium alginate solution containing sodium carbonate and 1.7g of crude silver iodide.
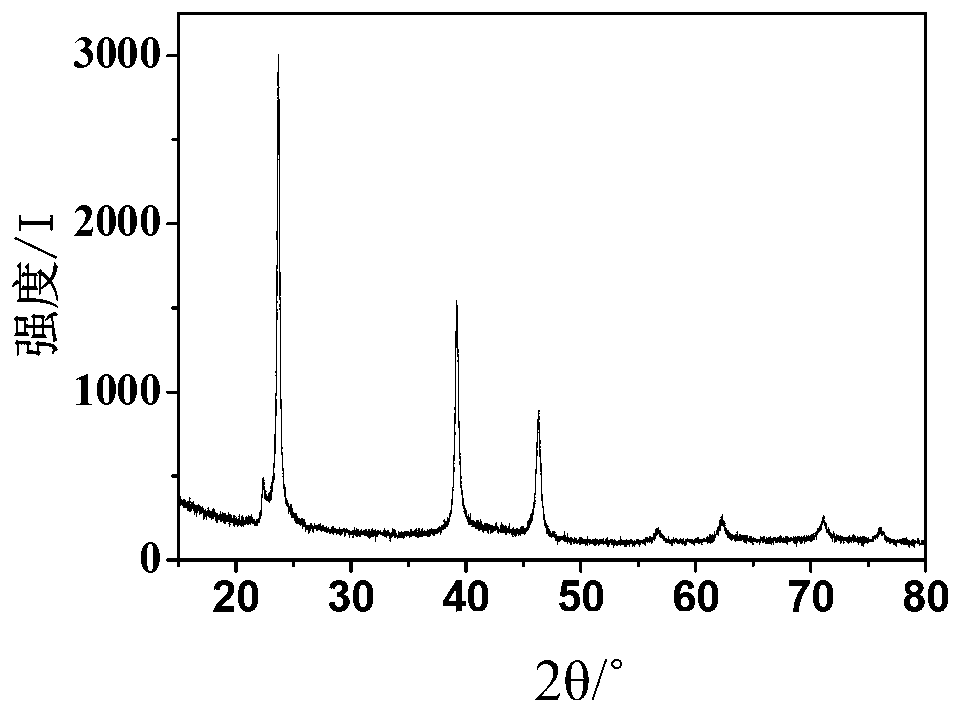
[0041] Purification of silver iodide: the obtained crude silver iodide was first washed 4 times with 2g 2wt% hydrochloric acid aqueous solution, and then washed 2 times with 15g water; the washed silver iodide solid wa...
Embodiment 2
[0045] Preparation of gel balls: Mix 250g of 2wt% sodium alginate aqueous sol with 1g of silver chloride solid powder to form a sol mixture; drop the sol mixture into 5wt% calcium chloride aqueous solution to produce a gel precipitate; The gel precipitate was separated from the liquid phase system to obtain 7 g of gel spheres.
[0046] Adsorption of iodine ions: put the gel balls into 22 kg of brine containing 40 mg / L iodine, stir and adsorb for 36 hours to obtain 8 g of iodine-loaded gel balls.
[0047] Preparation of silver iodide: Put 8 g of iodine-loaded gel balls into 50 g of 3 wt % excess sodium oxalate aqueous solution, stir thoroughly for 30 minutes, and filter to obtain sodium alginate solution containing sodium oxalate and 1.7 g of crude silver iodide.
[0048] Purification of silver iodide: the obtained crude silver iodide was first washed 4 times with 2g 2wt% hydrochloric acid aqueous solution, and then washed 2 times with 15g water; the washed silver iodide solid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com