Method for preparing abiraterone acetate without heavy-metal residue
A technology of abiraterone acetate and acid-binding agent, which is applied in the production of steroids, bulk chemicals, organic chemistry, etc., can solve the problems of unsuitability for large-scale production, harsh operation methods, unfavorable material preparation and operation, etc., and achieve good Prospect of industrial application and effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
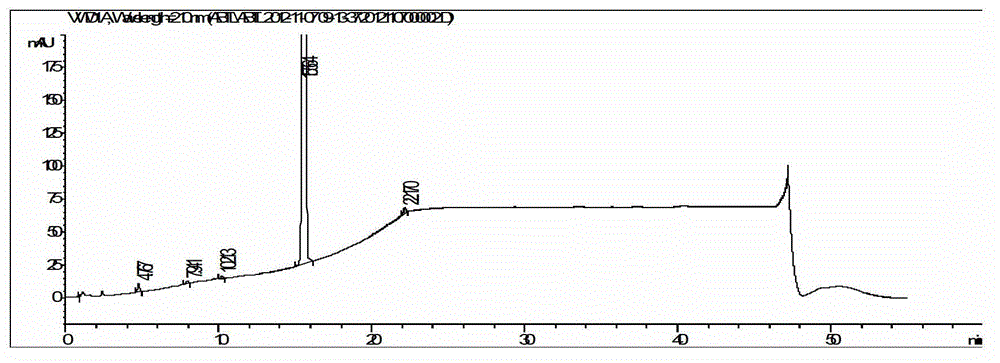
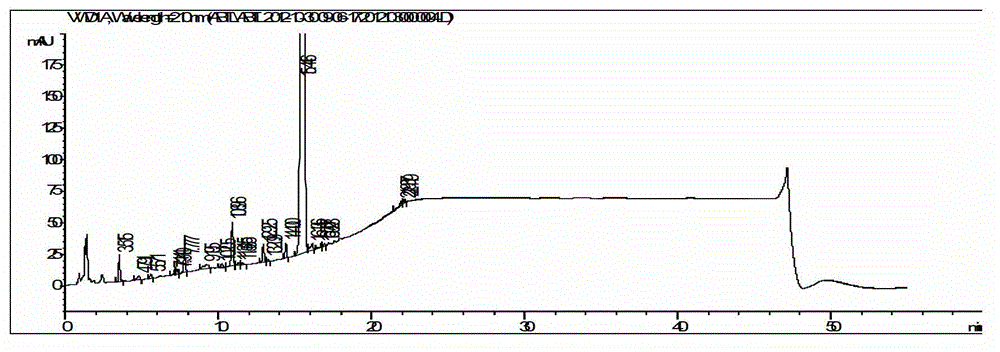
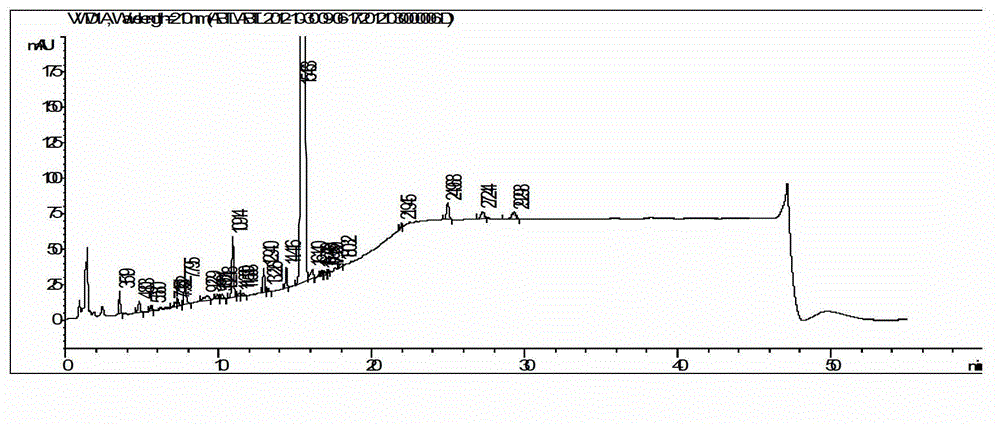
Image
Examples
Embodiment 1
[0045] The synthesis of embodiment 1 abiraterone acetate
[0046] (1) Hydroxyl protection
[0047] Dissolve 10.0 g of dehydroepiandrosterone in 80 ml of THF, add 3.5 g of 3,4-dihydro-2H-pyran (DHP) under stirring at room temperature, and add 0.5 ml of trifluoroacetic acid as a catalyst, and stir the reaction solution at room temperature for 10- 12h.
[0048] The reaction solution was desolvated under reduced pressure, the residue was dissolved in 100ml of water and 100ml of ethyl acetate, separated, the water phase was extracted twice with 50ml of ethyl acetate each time, the ethyl acetate phase was combined, washed once with 150ml of water, 150ml of saturated brine was washed once, dried with anhydrous sodium sulfate, and then precipitated to obtain 11.5g of the product (yield 89%), which can be directly used in the next step without purification.
[0049] (2) Aldol reaction
[0050] 10.0 g of THP-protected dehydroepiandrosterone was dissolved in 50 ml of THF after anhydro...
Embodiment 2
[0061] The synthesis of embodiment 2 abiraterone acetate
[0062] (1) Hydroxyl protection
[0063] Dissolve 10.0 g of dehydroepiandrosterone in 80 ml of THF, add 4.5 g of trimethylchlorosilane (TMSCl) while stirring at room temperature, and add 7.5 ml of triethylamine as an acid-binding agent, and stir the reaction solution at room temperature for 10-12 hours.
[0064] The reaction solution was desolvated under reduced pressure, the residue was dissolved in 100ml of water and 100ml of ethyl acetate, separated, the water phase was extracted twice with 50ml of ethyl acetate each time, the ethyl acetate phase was combined, washed once with 150ml of water, Wash once with 150ml of saturated saline, dry with anhydrous sodium sulfate, and remove the solvent to obtain 12.1g of the product (yield: 97%), which can be directly used in the next step without purification.
[0065] (2) Aldol reaction
[0066] 10.0 g of THP-protected dehydroepiandrosterone was dissolved in 50 ml of THF aft...
Embodiment 3
[0077] The synthesis of embodiment 3 abiraterone acetate
[0078] (1) Hydroxyl protection
[0079] Dissolve 10.0 g of dehydroepiandrosterone in 80 ml of DMF, add 6.3 g of trimethylchlorosilane (TBSCl) while stirring at room temperature, and add 3.3 g of imidazole as an acid-binding agent, and stir the reaction solution at room temperature for 10-12 hours.
[0080] The reaction solution was poured into 100ml of water, the phases were extracted three times with 50ml of ethyl acetate each time, the ethyl acetate phases were combined, washed once with 150ml of water, once with 150ml of saturated brine, dried with anhydrous sodium sulfate, and desolvated to obtain The crude product was 13.5g, which was quickly purified by thin-layer silica gel cake to obtain 12.8g (yield 92%), which could be used in the next reaction.
[0081] (2) Aldol reaction
[0082] 12.0 g of THP-protected dehydroepiandrosterone was dissolved in 50 ml of THF after anhydrous treatment, and pre-cooled for use....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com