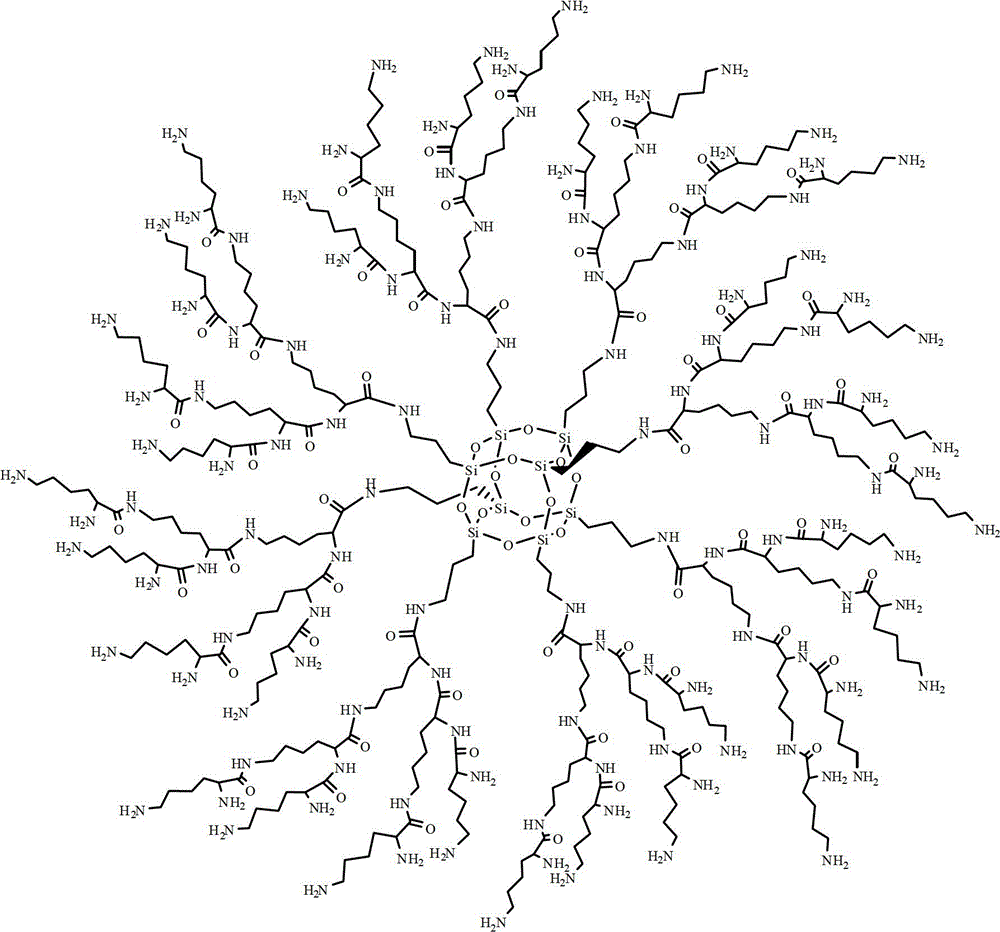
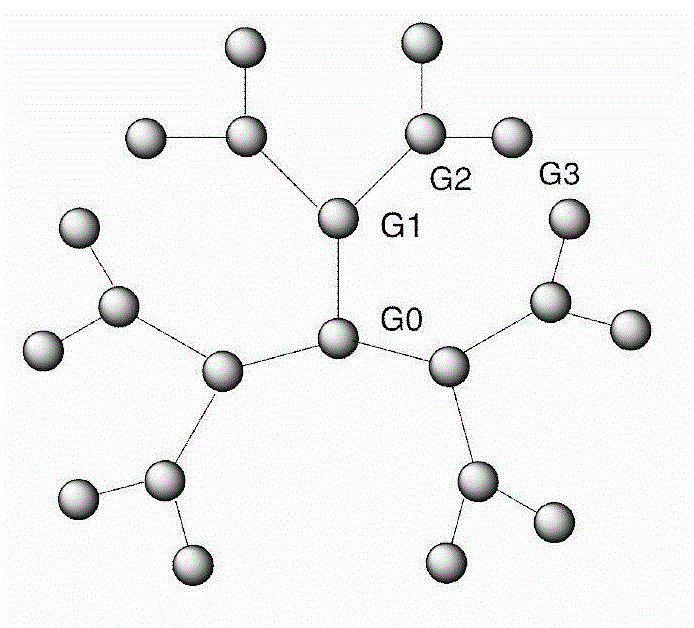
Synthesis method of dendriform compound trifluoroacetate using cage-type octamer (gamma-aminopropyl)silsesquioxane as core
A technology of trifluoroacetate and silsesquioxane, which is applied in the field of synthesis of dendrimer trifluoroacetate (G3(OL) trifluoroacetate), achieves less pollution, less species and higher yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Dissolve OAS hydrochloride (0.50g, 0.43mmol) and DIPEA (0.89g, 6.88mmol) in 20ml DMF, stir for 30min, then add N,N'-di-tert-butoxy in 30ml DMF drop by drop Carbonyl-L-lysine N-succinimide ester (3.05g, 6.88mmol), stirred at room temperature for 12 hours. After the reaction was completed, 150ml of acetonitrile chilled to 0°C was added, filtered, and the resulting solid was vacuum-dried at room temperature for 24 hours to obtain tert-butoxycarbonyl-protected G1(OL) (1.21g, 0.34mmol), with a yield of 80%.
[0024] 2. Add tert-butoxycarbonyl-protected G1(OL) (1.02g, 0.29mmol) into trifluoroacetic acid (5ml) chilled to 0°C and stir for 4 hours. After the reaction was completed, the reaction mixture was added to 50 ml of diethyl ether cooled to 0°C, and the precipitate was collected by filtration to obtain 0.80 g (0.21 mmol) of G1(OL) trifluoroacetate, with a yield of 74%.
[0025] 3. Using G1(OL) trifluoroacetate as raw material, adjust the molar ratio of G1(OL), DIPEA a...
Embodiment 2
[0028]1. Dissolve OAS hydrochloride (0.50g, 0.43mmol) and DIPEA (0.89g, 6.88mmol) in 20ml DMF, stir for 30min, then add N,N'-di-tert-butoxy in 30ml DMF drop by drop Carbonyl-L-lysine pentafluorophenol ester (3.52g, 6.88mmol), stirred and reacted at room temperature for 8 hours. After the reaction was completed, 150ml of acetonitrile chilled to 0°C was added, filtered, and the resulting solid was vacuum-dried to obtain tert-butoxycarbonyl-protected G1(OL) (1.28g, 0.37mmol), with a yield of 85%.
[0029] 2. Add tert-butoxycarbonyl-protected G1(OL) (1.02g, 0.29mmol) into trifluoroacetic acid (5ml) chilled to 0°C and stir for 4 hours. After the reaction was completed, the reaction mixture was added to 50 ml of diethyl ether cooled to 0°C, and the precipitate was collected by filtration to obtain 0.86 g (0.23 mmol) of G1(OL) trifluoroacetate, with a yield of 80%.
[0030] 3. Using G1(OL) trifluoroacetate as raw material, the molar ratio of G1(OL) trifluoroacetate, DIPEA and N,N-di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com