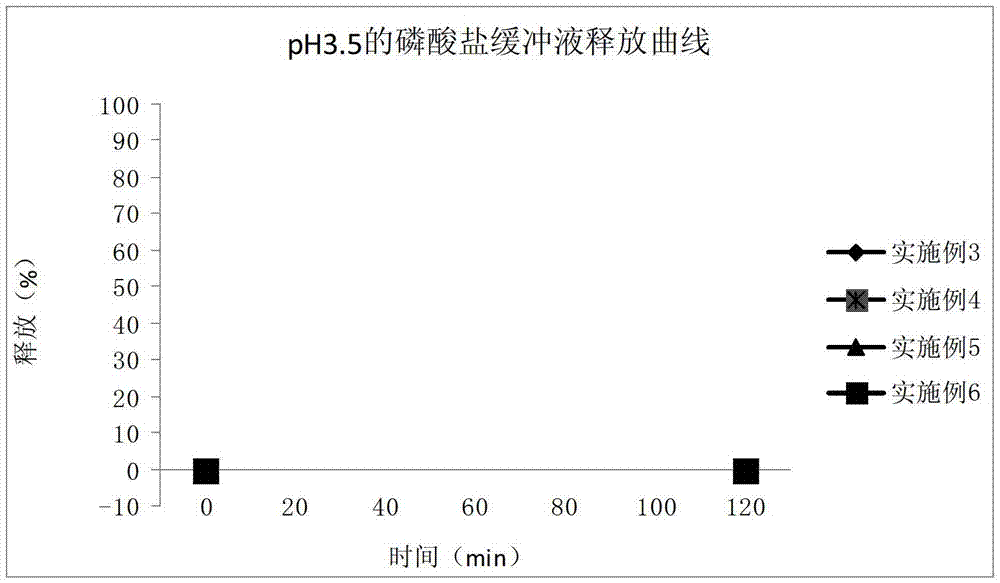
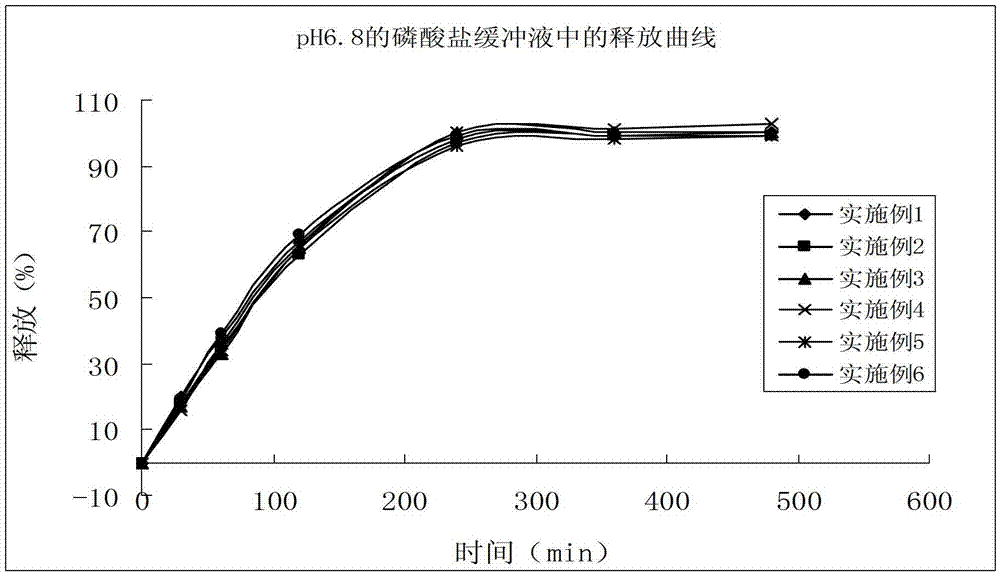
Enteric coated tablet of fenofibric acid and physiologically acceptable salts and preparation method of enteric coated tablet
A technology of fenofibric acid and physiology, applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of preparation method of fenofibric acid enteric-coated sustained-release tablets not mentioned , Suspensions are unfavorable for patients to take, and the dosage is not easy to control, etc., to achieve good effects, high production efficiency, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Among them, 178.69mg choline fenofibric acid is equivalent to 135mg fenofibric acid.
[0049] Preparation:
[0050]Weigh each material in the prescription, and mix the weighed choline fenofibric acid, hypromellose (K100LV), hypromellose (E50) and lactose in a granulator evenly. Spray 95% ethanol into the granulator during stirring. The prepared soft material is sieved through a granulator, dried, and the moisture content of the granules is controlled below 3%. Add the prescribed amount of magnesium stearate into the above-mentioned materials, and mix well. The tablets are punched with 9mm dimples to obtain sustained-release tablets.
Embodiment 2
[0053] Among them, 59.57mg of choline fenofibric acid is equivalent to 45mg of fenofibric acid.
[0054] Preparation:
[0055] Weigh each material in the prescription, and mix the weighed choline fenofibric acid, hypromellose (K100LV), hypromellose (E50) and lactose in a granulator evenly. Spray 95% ethanol into the granulator during stirring. The prepared soft material is sieved through a granulator, dried, and the moisture content of the granules is controlled below 3%. Add the magnesium stearate of prescription quantity in the above-mentioned material, mix well. The tablets are punched with 6mm dimples to obtain sustained-release tablets.
Embodiment 3
[0057] Tablet prescription:
[0058] material
mg / tablet
Proportion
178.69
59.57%
Hypromellose (K100LV) (sustained release material)
37
12.33%
Hypromellose (E50) (sustained release material)
31.5
10.50%
Lactose (filler)
49.7
16.57%
Magnesium Stearate (Lubricant)
3.1
1.03%
total
300.0
100%
[0059] Enteric Coating Prescription
[0060] Acryl (enteric coating material)
73.44g
water
293.76g
Coating weight gain about 15%
[0061] Preparation of enteric coating solution: Add Acryl to the stirring water, keep stirring until fully mixed and set aside.
[0062] Preparation:
[0063] Weigh each material in the prescription, and mix the weighed choline fenofibric acid, hypromellose (K100LV), hypromellose (E50) and lactose in a granulator evenly. Spray 95% ethanol into the granulator during stirring. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com