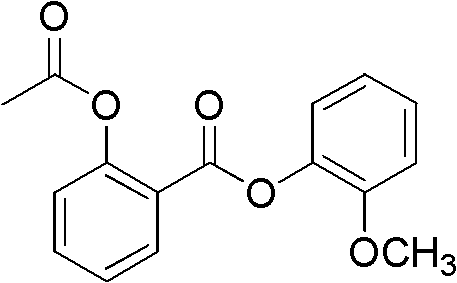
Industrialized preparation method of guacetisal and medical application of dry suspension
A technology of guacityl and catalyst, which is applied in the field of preparation of guacityl, can solve the problems of lowering system temperature by external circulation of cold water, high reaction temperature, long reaction time, etc., and achieves lower system temperature, mild reaction conditions, and production short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
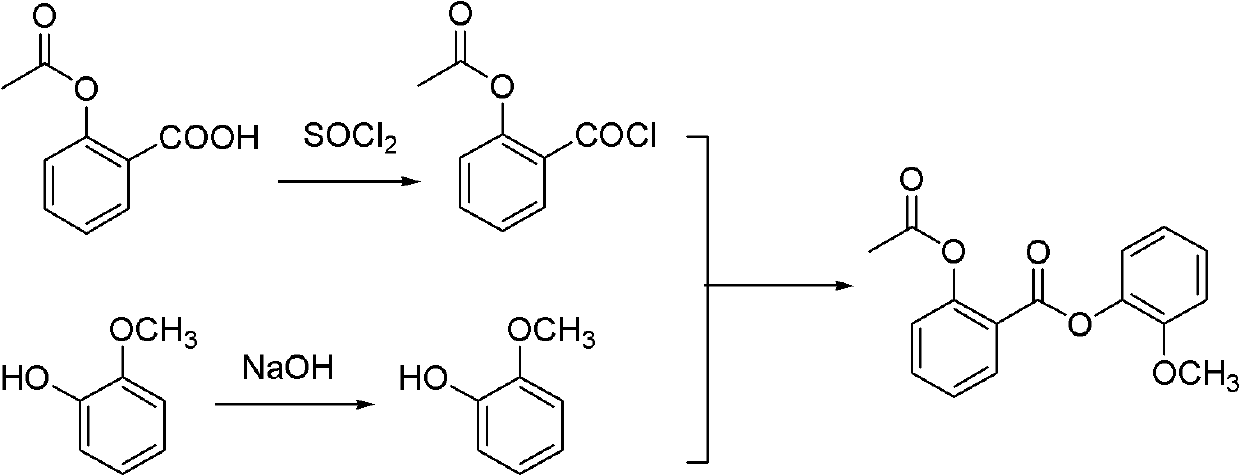
[0037] The first step: synthesis of guaiacol salicylate
[0038] Salicylic acid (200g, 1.45mol, 1.0eq) and guaiacol (180g, 1.45mol, 1.0eq) and DMAP (3.54g, 0.029mol, 2mol%) were added in the reactor, heated to 60±2 ℃ (at present existing reaction temperature is about 105 ℃, the present invention reduces the reaction temperature to 60 ℃ by adding catalyst, greatly reduces energy consumption, reduces cost), and phosphorus oxychloride (104.5g, 0.68mol, 0.47eq) was slowly dropped into the reaction solution (temperature control 65±2°C). After dripping, keep stirring and react for 3h. Add ethanol (300mL) dropwise, cool to 10-20°C, precipitate a solid, filter, wash the filter cake with ethanol (200mL), and vacuum-dry the filter cake at 45°C for 4h to obtain guaiacol salicylate, a white solid ( 311.2g, purity 98.5%, yield 88%).
[0039] The second step: the synthesis of Guaxitilian
[0040] Guaiacyl salicylate (311.2 g, 1.27 mol, 1.0 eq), acetic anhydride (156.1 g, 1.53 mol, 1.2 e...
Embodiment 2
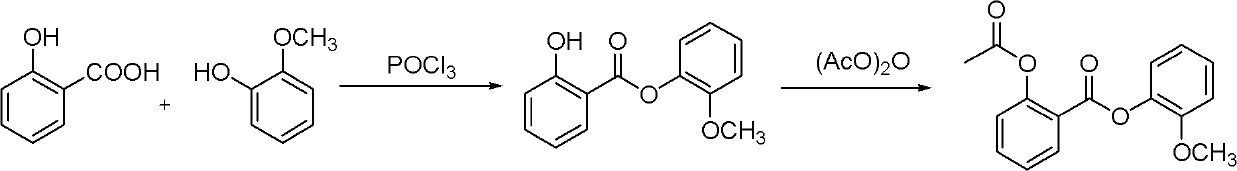
[0042] The first step: synthesis of guaiacol salicylate
[0043]Salicylic acid (1kg, 7.24mol, 1.0eq) and guaiacol (944g, 7.6mol, 1.05eq) and pyridine (17.4g, 0.22mol, 3mol%) were added in the reactor, heated to 60±2 °C, slowly drop phosphorus oxychloride (555 g, 3.62 mol, 0.5 eq) into the reaction solution (temperature control 65±2 °C). After dripping, keep stirring and react for 3h. Drop in isopropanol (1.2L), cool to 10-20°C, precipitate solid, filter, wash the filter cake with isopropanol (1L), and vacuum-dry the filter cake at 45°C for 4 hours to obtain guaiacol salicylate Ester, white solid (1.5kg, purity 98.8%, yield 85%).
[0044] The second step: the synthesis of Guaxitilian
[0045] Guaiacyl salicylate (1.5kg, 6.14mol, 1.0eq), acetic anhydride (627.0g, 6.14mol, 1.0eq) and DMF (561.2g, 7.68mol, 1.25eq) were added to the reactor, Heat to 60-65°C, keep stirring and react for 2 hours. Control the temperature at 50-60°C, add methanol (3L) into the reaction liquid, sti...
Embodiment 3
[0047] The first step: synthesis of guaiacol salicylate
[0048] Salicylic acid (5kg, 36.2mol, 1.0eq) and guaiacol (5.39kg, 43.44mol, 1.2eq) and DMF (132.3g, 1.81mol, 5mol%) were added in the reactor, heated to 60± At 2°C, phosphorus oxychloride (4.44kg, 28.96mol, 0.8eq) was slowly dropped into the reaction solution (temperature controlled at 65±2°C). After dripping, keep stirring and react for 3h. Add n-propanol (6L) dropwise, cool to 10-20°C, precipitate solids, filter, wash the filter cake with n-propanol (4L), and vacuum-dry the filter cake at 45°C for 4 hours to obtain guaiacol salicylate , white solid (7.96kg, purity 98.3%, yield 90%).
[0049] The second step: the synthesis of Guaxitilian
[0050] Guaiacyl salicylate (7.96kg, 32.6mol, 1.0eq), acetic anhydride (4.99kg, 48.9mol, 1.5eq) and DMAP (6.77kg, 55.4mol, 1.7eq) were added to the reactor, Heat to 60-70°C, keep stirring and react for 2 hours. Control the temperature at 50-60° C., add ethanol (15 L) into the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com