Brevibacterium and hydrolytic synthesis method of alpha-cyclo hexyl mandelic acid through nitrile and derivative
A technology of cyclohexylmandelic acid and Brevibacterium, applied in the field of microorganisms, achieves the effects of mild reaction conditions, no by-products, high conversion rate, and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
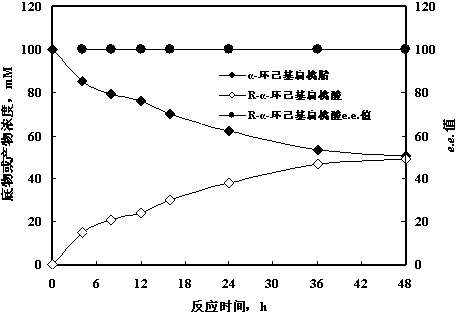
[0023] Brevibacterium Sp. CCZU12-1 quiescent cells catalyze the hydrolysis of α-cyclohexylmandelonitrile to produce α-cyclohexylmandelic acid
[0024] Prepare seed medium (glucose 5 g, peptone 5 g, yeast extract 5 g, KH 2 PO 4 1 g, NaCl 0.1 g, MgSO 4 0.1 g, inducer phenylacetonitrile 0.1 g, water 1000 mL, pH 6.0), fill 250 mL medium in a 1 L shake flask, and culture at 160 rpm and 25 °C Brevibacterium sp. CCZU12-1 for 48 h, centrifuged and washed to obtain resting cells. Weigh 0.1 g of resting cells with a wet weight, suspend the cells in 1.0 mL of pH 6.0 potassium phosphate buffer solution, add α-cyclohexylmandelonitrile to a final concentration of 100 mM, shake at 25 °C and 120 rpm on a constant temperature shaker reaction. Such as figure 1 Shown, 48 h R - The analytical yield of α-cyclohexylmandelic acid was 49.3%, e.e. >99.9%.
[0025]
Embodiment 2
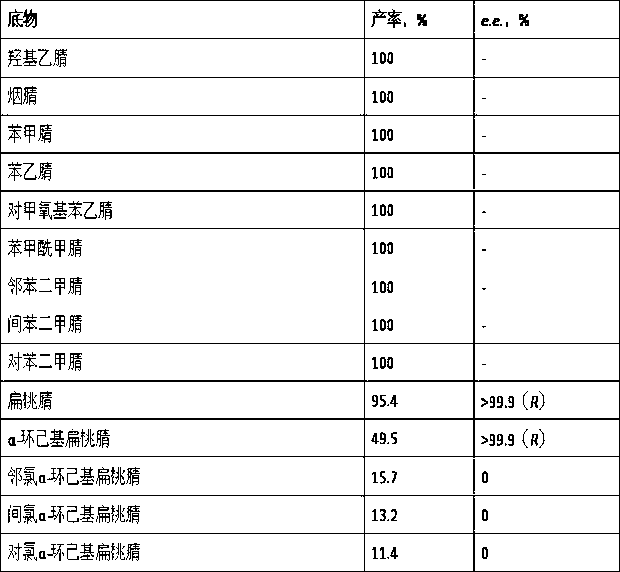
[0027] Brevibacterium Catalysis of different nitriles and their derivatives by sp. CCZU12-1 resting cells
[0028] Prepare medium (glucose 20 g, peptone 20 g, yeast extract 20 g, KH 2 PO 4 5 g, NaCl 1.5 g, MgSO 4 0.5 g, inducer phenylacetonitrile 1 g, water 1000 mL, pH 9.0), fill 250 mL medium in a 1 L shake flask, and culture at 160 rpm and 35 °C Brevibacterium sp. CCZU12-1 for 48 h, centrifuged and washed to obtain resting cells. Weigh 0.01 g resting cells with a wet weight, suspend the cells in 1.0 mL pH 8.0 potassium phosphate buffer solution, add different substrate nitriles to a final concentration of 20 mM, shake at 35 °C and 160 rpm on a constant temperature shaker After reacting for 12 h, the product results are shown in Table 2.
[0029]
[0030] Table 2 Expansion of the substrate spectrum
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com