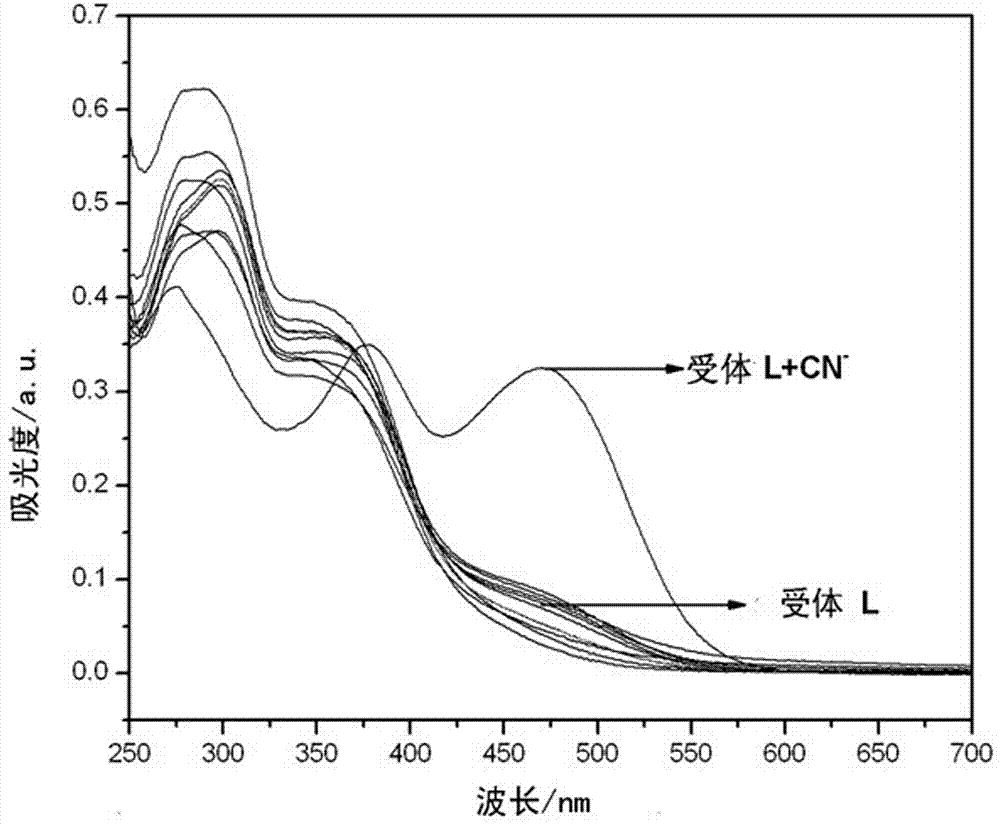
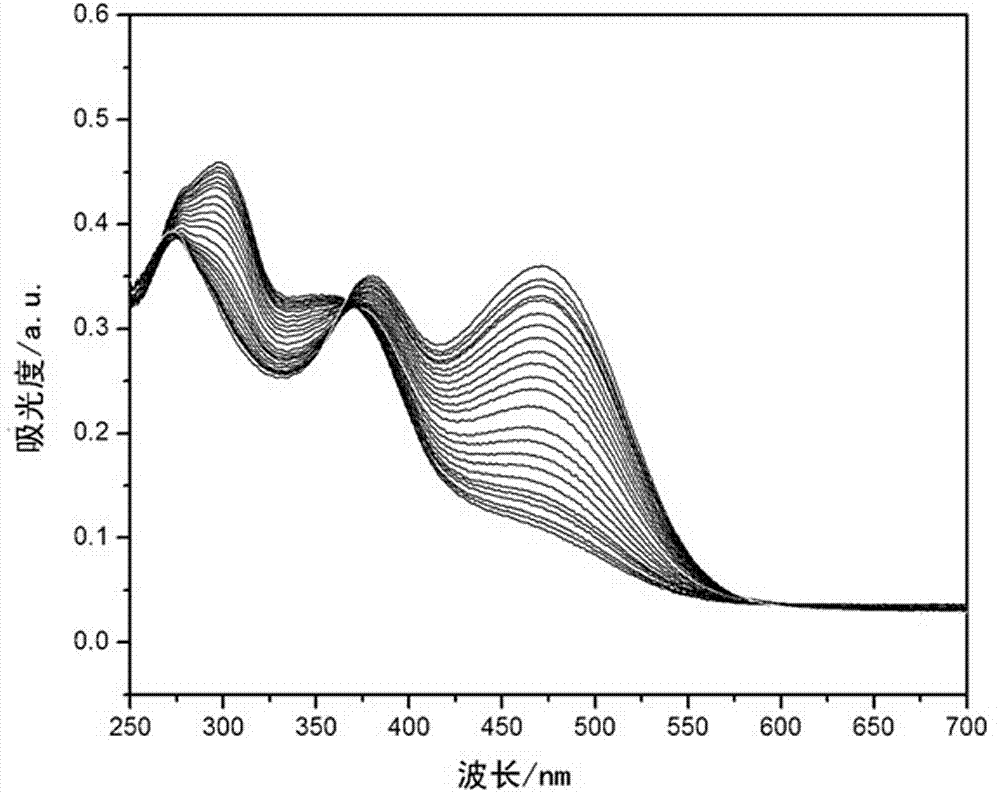
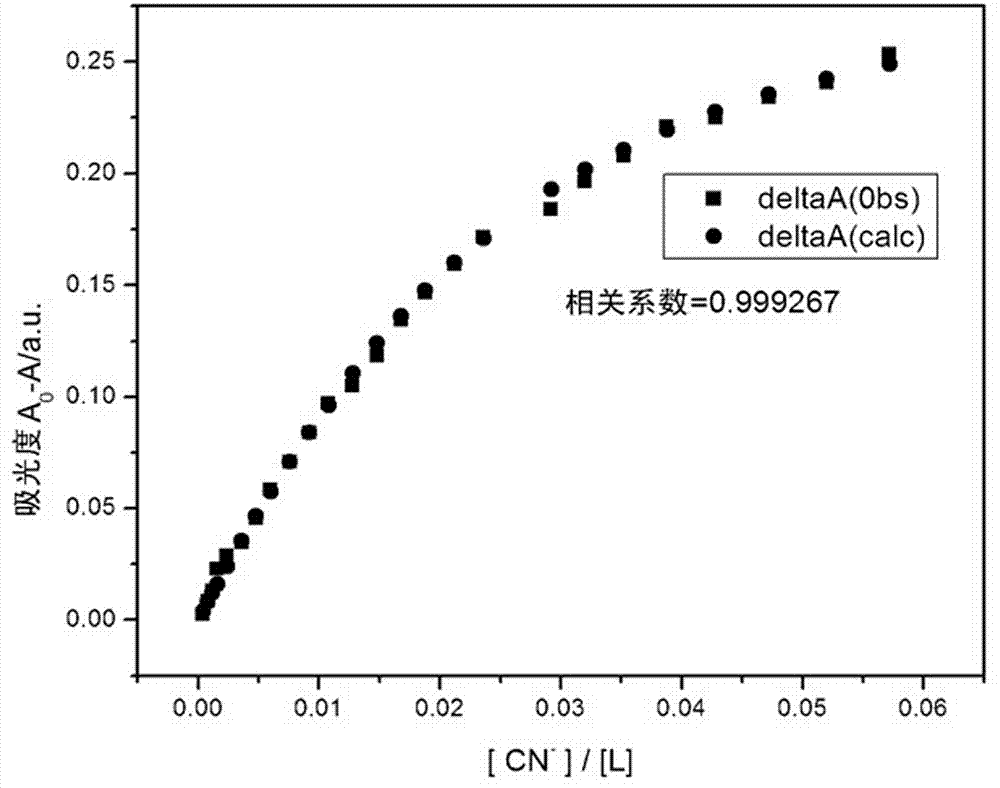
O-nitrophenyl azo salicylaldehyde phenoxy acetyl hydrazone, and synthesis and application thereof in CN<-> colorimetric detection
A kind of technology of nitrophenyl azo salicylaldehyde phenoxyacetyl hydrazone, nitrophenyl azo salicylaldehyde, applied in the field of organic chemical synthesis, can solve problems such as expensive, laborious, time-consuming and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Synthesis of receptor compound: Weigh 270 mg (1 mmol) of o-nitrophenyl azosalicylaldehyde and 166 mg (1 mmol) of phenoxyacetylhydrazine, and mix them in a 50 mL reaction flask; Add 20 mL of absolute ethanol as a solvent, 0.2 mL of glacial acetic acid as a catalyst, heat and reflux in an oil bath at 80°C for 8 h, a precipitate is formed, stand still, filter with suction, and use DMF-C 2 h 5 OH (DMF:C 2 h 5 OH=1:1) recrystallization to obtain the purple target product. Yield: 78%.
[0043] m.p.: 203-205°C. 1 H NMR (DMSO- d 6 , 400 MHz ppm) δ: 12.937 (s. 1H. OH), 10.14 (s. 1H. NH), 8.96 (s. 1H. N=CH), 6.944-6.987 (m. 4H. Ar–CH); IR (KBr, cm -1 ) ν: 1602(C=N); 1186(C=S); 2980(N=C–H); 3445(N–H). MS / (EI): m / z 420.2(M+H) + . Anal. Calc. for C 21 h 17 N 5 o 5 : C, 59.97; H, 4.05; N, 16.66. Found: C, 59.88; H, 4.12; N, 16.45.
[0044] 2. Production of test paper
[0045] Soak the filter paper in dilute hydrochloric acid with a mass concentration of 9.5-10% for ...
Embodiment 2
[0049] 1. Synthesis of acceptor compound: Weigh 270 mg (1 mmol) of o-nitrophenylazosalicylaldehyde and 332 mg (2 mmol) of phenoxyacetylhydrazine, and mix them in a 100 mL reaction flask; Add 50 mL of absolute ethanol as a solvent, 0.5 mL of glacial acetic acid as a catalyst, heat and reflux in an oil bath at 75°C for 8 h, a precipitate is formed, stand still, filter with suction, and use DMF-C 2 h 5 OH (DMF:C 2 h 5 OH=1:2) recrystallization to obtain the purple target product. Yield: 79%.
[0050] 2, the making of detection test paper: with embodiment 1.
[0051] 3. Test paper test CN - : with embodiment 1.
Embodiment 3
[0053] 1. Synthesis of acceptor compound: Weigh 270 mg (1 mmol) of o-nitrophenylazosalicylaldehyde and 249 mg (1.5 mmol) of phenoxyacetylhydrazine, and mix them in a 100 mL reaction flask; Add 50 mL of absolute ethanol as a solvent, 0.3 mL of glacial acetic acid as a catalyst, heat and reflux in an oil bath at 80°C for 7 h, a precipitate forms, stand still, filter with suction, and use DMF-C 2 h 5 OH (DMF:C 2 h 5 OH=1:1.5) recrystallization to obtain the purple target product. Yield: 76%.
[0054] 2, the making of detection test paper: with embodiment 1.
[0055] 3. Test paper test CN - : with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com