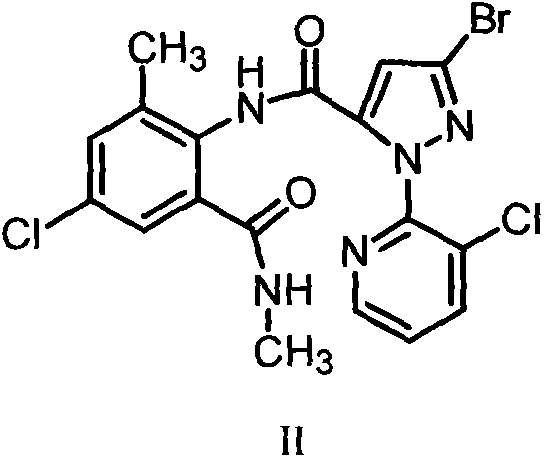
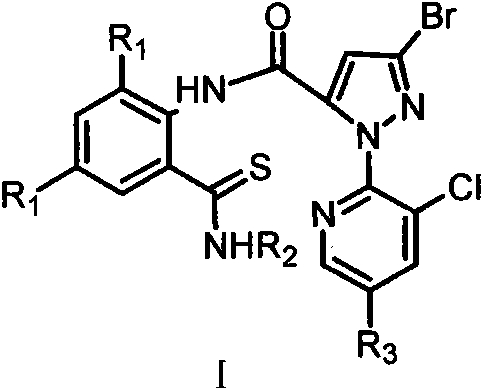
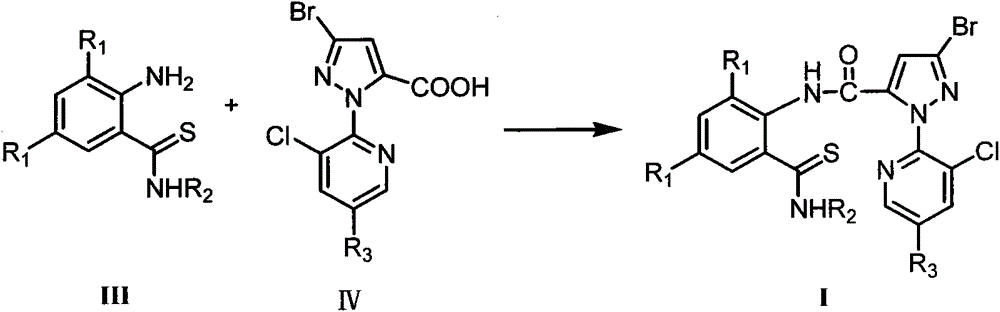
3,5-dihalothiobenzamide insecticides
A technology of dihalogenated thiobenzamide and insecticide, which is applied in 3 fields, can solve problems such as unpublished insecticidal activity, and achieve the effects of good comprehensive performance, low synthesis cost, and wide source of production raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] The preparation of example 1 compound 1
[0025] (1) Preparation of 2-amino-N-methylbenzamide
[0026]
[0027] In a 250mL reaction flask, add 16.3g (0.1mol) of isatoic anhydride, 100mL of ethyl acetate, 1mL of glacial acetic acid, add 15.5g (0.15mol) of 40% methylamine aqueous solution dropwise under stirring at room temperature, continue stirring for 2 hours, The disappearance of raw materials was detected by layer chromatography (TLC), and ethyl acetate and water were distilled off to obtain 13.2 g of white solid with a yield of 88%.
[0028] (2) Synthesis of 2-amino-3,5-dichloro-N-methylbenzamide
[0029]
[0030] In a 250mL reaction flask, add 15g (0.1mol) of 2-amino-N-methylbenzamide, add 80mL of acetonitrile, control the temperature in an ice bath below 10°C, slowly add 33.75g (0.25mol) of sulfonyl chloride dropwise, dropwise over 30 minutes Complete, stir at room temperature for 3h, evaporate most of the acetonitrile under reduced pressure, and use 20% N...
example 2
[0037] The preparation of example 2 compound 10
[0038] (1) Preparation of 2-amino-N-isopropylbenzamide
[0039]
[0040] In a 250mL reaction flask, add 16.3g (0.1mol) isatoic anhydride, 100mL ethyl acetate, 1mL glacial acetic acid, raise the temperature to 40-50°C, add 8.85g (0.15mol) of isopropylamine dropwise, continue stirring for 2h, and detect by TLC The raw materials disappeared, and ethyl acetate and water were distilled off to obtain 16.9 g of white solid, with a yield of 94.9%.
[0041] (2) 2-amino-3, the preparation of 5 dibromo-N-isopropylbenzamides
[0042]
[0043] In a 250mL reaction flask, add 17.8g (0.1mol) of 2-amino-N-isopropylbenzamide, 100mL of glacial acetic acid, add 32g (0.2mol) of bromine dropwise at room temperature, continue stirring for 3h, suction filter, filter Saturated Na 2 CO 3 After washing with aqueous solution and water, 32.4 g of white solid was obtained, with a yield of 96.3%.
[0044] (3) Preparation of 2-amino-3,5 dibromo-N-i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com